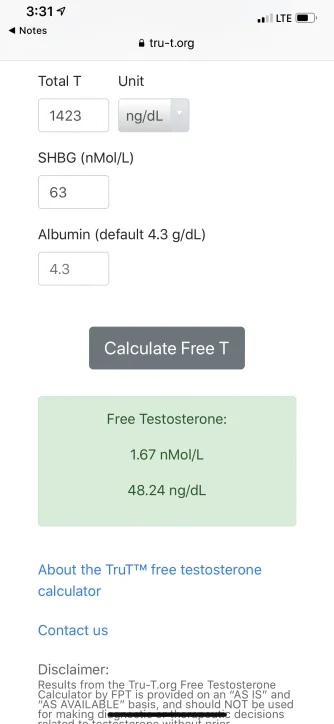
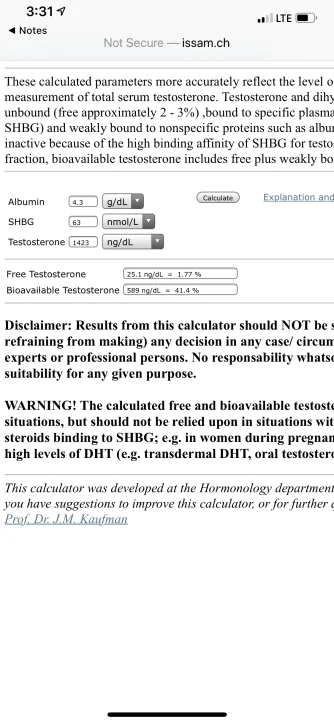
Seems like that method and the tru T method have drastically different results. I wonder why the difference between the two is so great. The tru T method is basically double the other method. That’s a huge difference.
Here is a detailed explanation: long but well worth the read!
* highlighted in green-
refer to the linear law-of-mass-action model/equation Vermueulen (cFTV)
*highlighted in blue-
refer to the new Multi-step Dynamic Binding Model with Complex Allostery (TruT calculated)
Methods and systems for the diagnosis and treatment of androgen disorders
Abstract
The technology described herein is directed to the diagnosis and treatment of androgen disorders and/or deficiencies, e.g. low testosterone.
Example 1
Determination of Free Sex Steroid Concentration
[0294] In conditions characterized by alterations in sex-hormone binding globulin (SHBG), total testosterone (TT) may not reflect the androgen status accurately and estimates of free testosterone (FT) are needed. Equations based on the prevailing model of SHBG:T interaction, although known to be systematically erroneous, have been used widely to calculate free testosterone.
[0295]Methods
[0296] Free testosterone concentrations calculated using a law-of-mass-action equation differ substantially from those measured using equilibrium dialysis. We investigated the dynamics of testosterone and SHBG interaction using binding isotherms, SHBG depletion curves, and isothermal titration calorimetry (ITC). The FT values calculated from new model derived from these experiments were compared to equilibrium dialysis using samples from randomized testosterone trials in men and women.
[0297] Results
[0298] Binding isotherms generated by incubating 5, 10 or 20 nM SHBG with 0-to-400 nM testosterone could not be explained by the prevailing model developed by Vermeulen et al. (1). Comprehensive evaluation of data derived from binding isotherms, ligand depletion curves, and ITC suggested a complex association of testosterone with SHBG dimer; the new Multi-step Dynamic Binding Model with Complex Allostery that provided the best fit to these data encompasses at least two inter-converting microstates in unliganded SHBG, readjustment of equilibria between unliganded states upon binding of the first ligand molecule, and allosteric interaction between two binding sites of SHBG dimer. In samples from testosterone trials in men and women, free testosterone predicted using the new Multi-step Dynamic Binding Model with Complex Allostery did not systematically differ from that measured using equilibrium dialysis.
[0299] The new Multi-step Dynamic Binding Model with Complex Allostery of testosterone's binding to SHBG provides excellent fit to experimental data derived from binding isotherm, depletion curves and ITC. Free testosterone concentrations calculated using the new Multi-step Dynamic Binding Model with Complex Allostery closely match those measured using equilibrium dialysis.
[0300] Testosterone, the major androgen in humans, circulates in blood bound largely to sex hormone binding globulin (SHBG) and albumin. Testosterone can also bind to orosomucoid and transcortin proteins. According to the free hormone hypothesis, only the unbound or free fraction—0.5 to 3.0% of total—can cross the plasma membrane and is biologically active. In many conditions that affect SHBG concentrations, such as obesity, diabetes, aging, hyperthyroidism, liver disease, acromegaly, and HIV-infection, total testosterone concentrations are altered because of the changes in SHBG concentrations; in these conditions, determination of free testosterone concentration is needed to obtain an accurate assessment of androgen status.
[0302] The current equations based on homogenous SHBG:T interaction (equal affinity of T for each of the monomers within SHBG dimer and without allostery in SHBG dimers) proposed by Vermeulen and others (13, 17) are based on the assumption that each SHBG dimer binds two testosterone molecules, and that each of the two binding sites on SHBG dimer has similar binding constants (data not shown). It is demonstrated herein that the current model of testosterone binding to SHBG that has formed the basis of the law-of-mass-action equation is erroneous; the free testosterone levels derived from these equations display substantial discrepancy from the values obtained by equilibrium dialysis (18). Based on binding isotherms, ligand depletion experiments, and isothermal titration calorimetry (ITC), we provide experimental evidence of complex allostery between the binding sites on the two SHBG monomers in the presence of the ligand. Based on this new model of testosterone binding to SHBG, described herein is a novel algorithm for the calculation of free testosterone, applied it to samples derived from randomized testosterone trials in men and women, and compared the results with those obtained using equilibrium dialysis.
[0303] Materials and Methods
[0304] Human SHBG purified from serum (The Binding Site Group, Ltd Birmingham, UK cat# BH089.X) was characterized by protein gel denaturation-renaturation experiments and by measuring its ability to bind testosterone. Testosterone standard 1.0 mg/mL±2% (3.47 mM) was obtained from Cerilliant (Round Rock, Tex.).
[0305] Equilibrium dialysis was performed in 96-Well equilibrium dialysis chambers with 10 kDa molecular weight cut-off (Harvard Apparatus, Holliston, Mass. cat#742331). Equilibrium dialysis buffer contained 30 mM HEPES buffer pH 7.4, 90 mM NaCl, 1 mM MgSO4, 187 uM CaCl2 in ultrapure water. For binding and depletion assays, SHBG was reconstituted in equilibrium dialysis buffer. Testosterone concentration was measured using a liquid chromatography tandem mass spectrometry (LC-MS/MS) assay that has been certified by the Center for Disease Control's HoST Program(19).
[0306] Isothermal calorimetry(ITC) experiments were performed using fully automated Auto-ITC200 calorimeter from MicroCal (Northampton, Mass.) provided by Automated Biological calorimetry Facility (Huck Institutes of Life Sciences, University Park, Pa.). SHBG was reconstituted in 30 mM HEPES buffer, pH 7.4, to a final concentration of 5 μM. Testosterone standard was prepared in DMSO and diluted in protein buffer to a concentration of 100 μM in 5% DMSO. DMSO was added to SHBG by weight to match DMSO content in the testosterone solution. Samples were degassed prior to loading to the calorimeter. Testosterone was injected into the protein solution in 32 equal steps. Heat produced by each injection was measured by the calorimeter. Interval between injections was set at 240 seconds so that the temperature could return to baseline. The amount of heat generated after each injection (after subtracting the heat of dilution of ligand in buffer) was integrated to produce calorimetric binding isotherm depicting the relationship of the total heat generated in the reaction to testosterone-to-SHBG molar ratio.
[0307] Application of the Novel Algorithm to Clinical Trials Data.
[0308] Free testosterone concentrations determined using the novel algorithm described herein and by the Vermeulen's law-of-mass-action equation, were compared (implemented in a spreadsheet by Mazer) against those measured using the reference method (equilibrium dialysis), in samples derived from randomized testosterone trials in men (101) and women (102). These samples had been collected in fasting state in the morning, stored immediately after collection at −80° C., and never thawed.
[0309] Testosterone in Men with Erectile Dysfunction (TED) Trial.
The TED Trial, whose design and results have been published (20), was a randomized, placebo-controlled trial to determine whether addition of testosterone to an optimized regimen of sildenafil citrate improves erectile function in men with erectile dysfunction (ED) and low testosterone levels. At baseline and after 12-weeks of testosterone or placebo administration, serum total testosterone concentrations were measured using LC-MS/MS and SHBG concentrations measured by a two-site immunofluorometric assay (DELFIA® SHBG Kit, cat# A070-101, Perkin-Elmer, Waltham, Mass.). Free testosterone concentrations were measured in the same samples by equilibrium dialysis.
Results
[0311] The free testosterone concentrations calculated by the Vermeulen's equation (24) were compared with those measured using equilibrium dialysis in samples derived from participants in the TED Trial. As shown in FIG. 1A, free testosterone concentrations estimated by this equation were significantly lower than those measured by equilibrium dialysis. Bland Altman plot (FIG. 1B) confirmed the substantial underestimation of free testosterone concentration by the Veinieulen's equation relative to that measured by equilibrium dialysis, although the estimation error was not linearly related to the measured free testosterone concentrations.
To determine the molecular basis of this discrepancy, we used three experimental approaches to characterize testosterone's binding to SHBG: the binding isotherms, the ligand depletion curve, and the isothermal titration calorimetry (ITC).
[0312] Testosterone: SHBG binding Isotherms display evidence of homoallosteric association between testosterone and SHBG dimers. To generate the binding isotherms, 5, 10 or 20 nM SHBG protein (dimer) was incubated with graded concentrations of testosterone (0 to 400 nM) at room temperature (20° C.). The mixture was dialyzed overnight against an equal volume of the dialysis buffer and testosterone concentration in the dialysate was determined by LC-MS/MS. When bound testosterone concentration was plotted against total testosterone concentration (FIG. 2A), the binding isotherm displayed several distinct features: the existence of two distinct saturation plateaus (including an apparent plateau at lower testosterone concentrations), and asymmetry of the isotherm around the EC50value. The relationship of bound testosterone concentration to total testosterone could not be adequately fit by the Vermeulen's model/equation, which assumes that the two monomers within the SHBG dimer have similar binding constant
[0313] The new Multi-step Dynamic Binding Model with Complex Allostery presented in this study was developed iteratively to encompass all the features of the binding isotherms. In an attempt to comprehensively fit binding isotherms, several models were examined: a) the prevailing equations suggesting a homogenous interaction of two testosterone molecules, b) the monomers within dimer exhibiting distinct affinity constants, c) simple allostery where binding of first ligand alters the affinity of the second site and
d) the new Multi-step Dynamic Binding Model with Complex Allostery including two distinct SHBG dimer microstates in equilibrium such that the equilibria between the unliganded and mono-liganded readjusts as the concentration of testosterone is increased. Consistent with the crystal structure data, all the models were constrained to eventually converge on to a single double liganded conformational state of SHBG dimer Of all the models tested, the new Multi-step Dynamic Binding Model with Complex Allostery adequately fit the binding isotherms. The schematic representation in FIG. 3 summarizes the complex dynamics of the T:SHBG interaction. In this model it was further tested if SIT and S1′T microstates were distinguishable or not. That model with converged SIT and S1′T states again fails to fit the data. The new Multi-step Dynamic Binding Model with Complex Allostery fit the binding isotherm optimally and explained the observed saturation plateaus, including the plateau at lower testosterone concentrations, and the asymmetry of the binding isotherm around the ES50.
[0314] Testosterone Depletion Curves.
[0315] As an independent and complementary assessment of testosterone's binding to SHBG, we incubated various amounts of SHBG (0.1 to 500 nM) with a fixed concentration of testosterone, and analyzed the depletion of unbound testosterone when increasing concentrations of SHBG were added. These depletion curves were generated at several different testosterone concentrations (6 nM, 12 nM, 18 nM, and 32 nM). Mixtures of testosterone and SHBG were dialyzed overnight against a similar volume of the dialysis buffer and free testosterone concentration was measured by LC-MS/MS. The relationship of free testosterone concentration to SHBG concentration in the depletion experiments (FIG. 2B) was again best fit using the new Multi-step Dynamic Binding Model with Complex Allostery. The Vermeulen model did not provide an optimum fit (data not shown).
[0316] Isothermal Titration Calorimetry (ITC).
[0317] To validate the new Multi-step Dynamic Binding Model with Complex Allostery further and to evaluate the thermodynamic parameters associated with testosterone binding to SHBG, ITC experiments were performed. The ITC isotherm has a characteristic shoulder (FIG. 2C) and cannot be adequately described as a simple sigmoidal curve predicted by the Vermeulen model nor by models B and C. Using the computational framework developed in LABVIEW (21), fits of the ITC data to the new Multi-step Dynamic Binding Model with Complex Allostery were generated (FIG. 3). Model constants obtained as a result of the linked fit in FIGS. 2A-2B were used as a starting point for the fit. The new Multi-step Dynamic Binding Model with Complex Allostery model provided an excellent fit for the experimental data derived from the ITC. The shape of the ITC curve can be explained as a convoluted result of testosterone binding and multiple conformational rearrangements defined by the new Multi-step Dynamic Binding Model with Complex Allostery. While, the independent enthalpy parameters for each of the individual reactions comprising the model were used, they are not simultaneously identifiable.
[0318] Application of the New Algorithm to Clinical Trials Data
[0319] The new model was applied to data generated in the TED trial (FIGS. 4A-4D). The free testosterone concentrations were calculated by the prevailing equations (23-24) and those calculated using the new algorithm based on new Multi-step Dynamic Binding Model with Complex Allostery (FIGS. 4A-4D). The prevailing model (17) significantly underestimated free testosterone levels relative to equilibrium dialysis in men participating in the TED trial. In contrast, the new Multi-step Dynamic Binding Model with Complex Allostery model provided values that were not statistically different from those measured by equilibrium dialysis (slope 1.01±0.01) in both men and women. The Bland-Altman plots (FIGS. 4A-4D) show the absence of any systematic difference between the values derived from the new Multi-step Dynamic Binding Model with Complex Allostery model and those obtained using the equilibrium dialysis in either men or women; the relative deviation of the values calculated using the new Multi-step Dynamic Binding Model with Complex Allostery model from those measured using equilibrium dialysis was evenly distributed around 0, likely reflecting multiple sources of measurement error in the testosterone assay, SHBG assay, and in the equilibrium dialysis method (FIGS. 4A-4D).
[0320] Discussion.
[0321] It is demonstrated herein that the current model of testosterone binding to SHBG that has formed the basis of the law-of-mass-action equations for several decades to estimate free testosterone concentrations is erroneous. While the discrepancy between testosterone concentrations estimated using the available prevailing equations and those measured using the equilibrium dialysis method has been recognized ( ), the present data provide a rational mechanistic explanation for this discrepancy that had remained obscure previously. The experimental data from the binding isotherms, the SHBG depletion curves, and the ITC cannot be explained by the existing SHBG-T interaction model of single binding site or two identical, non-interacting binding sites on SHBG. The experimental data are poorly explained even by simple models for homotropic allostery within a dimer (e.g., Koshland-Nemesy-Filmer (22) and Monod-Wyman-Changeaux (23) models. In contrast, the new Multi-step Dynamic Binding Model with Complex Allostery (24, 25) optimally fits the data from the binding isotherms and also explains adequately the depletion curves and the ITC data. Furthermore, in samples derived from male and female participants in two separate clinical trials, testosterone concentrations calculated using the new Multi-step Dynamic Binding Model with Complex Allostery were statistically not different from those measured by the equilibrium dialysis.
[0322] The proposed new Multi-step Dynamic Binding Model with Complex Allostery indicates that in the absence of testosterone, SHBG molecule can assume one of at least two inter-converting microstates in a dynamic equilibrium. The binding of testosterone to one of the monomers of the SHBG dimer in a given microstate affects the interaction of testosterone with the unoccupied second binding site on the SHBG dimer. The model suggests a dynamic readjustment of populations of intermediate species as testosterone concentrations are altered. Independent experimental validation of model was performed by generating ligand depletion and ITC studies. The data from three sets (equilibrium binding, ligand depletion and ITC) were successfully fit by the new Multi-step Dynamic Binding Model with Complex Allostery. Without wishing to be bound by theory, the parameters in the model are not uniquely determined and could require detailed experimental evaluation of each microstate in multiple equilibria.
[0323] Prevailing hypothesis that has formulated the basis of the equations/expressions by Vermeulen assumes that monomers within a dimer display identical association constant without any dynamic interaction between the subunits. The general idea was supported by ligand bound crystal structure of SHBG (36). Parenthetically, the crystal structures are typically obtained in saturating concentrations of ligands. It is possible that the inability to resolve the unliganded SHBG structure (26) as well as the increased stability of SHBG upon ligand binding (27) may be related to the significant rearrangement of SHBG molecule upon binding of the first ligand, as predicted by the new Multi-step Dynamic Binding Model with Complex Allostery (24). The additional energy barrier that SHBG has to overcome may result in altered affinity for binding of the second ligand molecule. Further studies utilizing dimerization deficient mutants as well as molecular dynamics simulations are required to precisely define biophysical parameters associated with each ligand binding event.
[0324] The algorithm based on new Multi-step Dynamic Binding Model with Complex Allostery was applied to human samples obtained from randomized trials in men and women. It was found that over a wide range of testosterone concentrations prevalent in the male and female participants in the two testosterone trials, the free testosterone concentrations determined using the new algorithm were similar to those measured using the equilibrium dialysis. Also, the algorithm was applicable over a wide range of testosterone and estradiol concentrations in men and women. Without wishing to be bound by theory, there is a possibility that very high estrogen concentrations, such as those that may be observed during pregnancy, may affect testosterone binding to SHBG, introducing potential error in the calculated concentrations.
[0325] The current algorithm and the experimental data reported here were generated using wild type SHBG which is present in nearly 98% of Caucasians. Genome wide association studies have revealed several SHBG polymorphisms, two of which have been reported to affect testosterone binding to SHBG (28). Therefore, in future, the algorithms may include a term for the SHBG genotype. In summary, the experimental data of testosterone's association with SHBG implicate a complex allostery mechanism within the SHBG dimer. The new Multi-step Dynamic Binding Model with Complex Allostery provides excellent fit to the experimental data generated using three different techniques. Unlike the existing equations based on homogenous binding of testosterone with SHBG, which reveal systematic discrepancy from the values obtained by equilibrium dialysis, the free testosterone concentrations derived using the new model do not differ significantly from those measured using equilibrium dialysis.
Example 2
A New Multi-Step Dynamic Binding Model with Complex Allostery of Testosterone's Binding to Sex Hormone Binding Globulin
[0354] Circulating free testosterone (FT) levels have been used widely in the diagnosis and treatment of hypogonadism in men. Due to experimental complexities in FT measurements, the Endocrine Society expert panel has recommended the use of calculated FT (cFT) as an appropriate approach for estimating FT.
It is demonstrated herein that the prevailing model of testosterone's binding to SHBG, which assumes that each SHBG dimer binds two testosterone molecules and that the two binding sites on SHBG have similar binding affinity, provides values of free testosterone that differ substantially from those obtained using equilibrium dialysis.
[0355] Described herein is the characterization of testosterone's binding to SHBG using equilibrium dialysis (binding isotherms varying both ligand and protein) and isothermal titration calorimetry. These experimental data were utilized in the development of a new model of testosterone's binding to SHBG; and this new model permitted the determination of free testosterone concentrations and comparison of these values to those derived from equilibrium dialysis.
[0366] Experimental data from equilibrium dialysis experiments, and isothermal titration calorimetry provide evidence of complex homoallostery within SHBG. Described herein is a New Multi-Step Dynamic Binding Model with Complex Allostery encompassing at least two inter-converting microstates in unliganded SHBG, readjustment of equilibria between unliganded states upon binding of the first ligand molecule, and allosteric interaction between two binding sites of SHBG dimer Free testosterone concentrations determined using framework incorporating intra-dimer allostery did not differ from those measured using equilibrium dialysis in samples from clinical trials Testosterone's binding to SHBG is demonstrated herein to be a multi-step process that involves complex homo-allostery within SHBG dimer cFT values obtained using the new model have close correspondence with those measured using equilibrium dialysis.
[0357] Introduction
[0358], the major androgen in humans, circulates in blood bound largely to sex hormone binding globulin (SHBG) and albumin (Rosner 1991, Hammond and Bocchinfuso 1996, Bhasin et al 2010, Mendel 1989, Rosner et al 2007). Testosterone can also bind to orosomucoid and transcortin proteins, but the amount of testosterone bound to these proteins in human plasma is negligible. According to the free hormone hypothesis, only the unbound or free fraction can cross the plasma membrane and is biologically active (Rosner 1991, Hammond and Bocchinfuso 1996, Bhasin et al 2010, Mendel 1989, Rosner et al 2007). In many conditions that affect SHBG concentrations, such as obesity, diabetes, aging, hyperthyroidism, liver disease, and HIV-infection, total testosterone concentrations are altered because of changes in SHBG concentrations; in these conditions, expert panels have recommended determination of free testosterone (FT) concentration to obtain an accurate assessment of androgen status (Rosner 1991, Hammond and Bocchinfuso 1996, Bhasin et al 2010, Mendel 1989, Rosner et al 2007).
[0359] The current model of testosterone's binding to SHBG assumes that each SHBG dimer binds two testosterone molecules, and that each of the two binding sites on SHBG dimer has similar binding affinity. Equations to determine FT were proposed by Vermeulen and others (Rosner et al 2007, Sodergard et al 1982, Vermeulen et al 1971, Mazer 2009)(Sodergard et al 1982, Vermeulen et al 1971). We show here that the prevailing model of testosterone's binding to SHBG is erroneous. The data from equilibrium dialysis and isothermal titration calorimetry (ITC) experiments provide evidence for ligand modulated allosteric interaction between the binding sites on the two SHBG monomers.
[0360] Reflecting the growing interest in men's health and the success of pharmaceutical advertising, testosterone sales have grown from 23 million dollars in 1993 to 70 million in 2000 to 1.7 billion dollars in 2012 (Spitzer et al 2012). Testosterone is the second most frequently ordered test, next only to 25-hydroxyvitamin D. In 2012, nearly 4 million free testosterone tests were performed in the USA alone. A number of direct and indirect methods—equilibrium dialysis, ultrafiltration, tracer analog methods, and calculations based on homogenous (equal affinity of testosterone for each monomer in SHBG dimer) binding—have been developed for the determination of FT levels (Rosner et al 2007, Sodergard et al 1982, Vermeulen et al 1971, Mazer 2009, Rosner 1997, Winters et al 1998, Vermeulen et al 1999, Sinha-Hikim et al 1998, Van Uytfanghe et al 2004, Adachi et al 1991, Morley et al 2002)(Sodergard et al 1982, Vermeulen et al 1971). Expert panels have expressed concern about the accuracy and methodological complexity of the available assays for FT (Rosner et al 2007, Sodergard et al 1982, Vermeulen et al 1971). Recognizing these methodological difficulties in the measurement of free testosterone, the Endocrine Society's Expert Panel suggested that “the calculation of free testosterone from reliably measured total testosterone and SHBG using mass action equations provides the best approach for the estimation of free testosterone . . . ” (Rosner et al 2007).
Therefore, algorithms for calculating FT from total testosterone, SHBG and albumin concentrations have been used widely (Rosner et al 2007, Mazer 2009, Morley et al 2002, Morales et al 2012, Ly et al 2010, Sartorius et al 2009, Bhasin et al 2011, Ly and Handelsman 2005). The equations are either based on the binding mechanism and law of mass-action (Sodergard et al 1982, Mazer 2009, Vermeulen et al 1999)(Sodergard et al 1982, Vermeulen et al 1999, Sartorius et al 2009, Ly and Handelsman 2005, Nanjee and Wheeler 1985) or are empirically-derived (Ly et al 2010, Sartorius et al 2009, Ly and Handelsman 2005, Nanjee and Wheeler 1985)(Sodergard et al 1982, Sartorius et al 2009, Ly and Handelsman 2005, Nanjee and Wheeler 1985).
[0361] Described herein is equilibrium dialysis (varying both SHBG and ligand concentrations) and isothermal titration calorimetry (ITC) to characterize testosterone's binding to SHBG. Various possible mechanistic models of molecular interactions, including linear homogeneous binding of testosterone to SHBG as envisioned by Vermeulen (Vermeulen et al 1999), Sodergard (Sodergard et al 1982) and Mazer (Mazer 2009), and various allosteric mechanisms, including allostery with positive and negative cooperativity (Koshland et al 1966, MONOD et al 1965), and an ensemble allosteric mechanism (Hilser and Thompson 2007) were considered. Based on our analyses of the experimental data of testosterone's binding to SHBG, a novel algorithm was constructed for the calculation of FT, which included intra-dimer allostery, which provided the best fit to the totality of experimental data. This new model was applied to determine free testosterone concentrations in samples derived from randomized testosterone trials, the results compared with those obtained using equilibrium dialysis.
[0362] Materials and Methods
[0363] Biophysical characterization. Human SHBG purified from serum (Binding Site Group, Birmingham, UK) was characterized by protein gel denaturation-renaturation experiments and by measuring its ability to bind testosterone. Testosterone concentration in the SHBG stock solution, measured using LC-MS/MS, was undetectable. Testosterone standard 1.0 mg/mL±2% (3.47 mM) was obtained from Cerilliant (Round Rock, Tex.).
[0364] Binding profiles were established by the equilibrium dialysis (varying either ligand or protein concentration) were performed in 96-well dialysis plates containing dialysis chambers separated by membranes with 10 kDa cut-off (Harvard Apparatus, Holliston, Mass.). SHBG and testosterone were reconstituted in dialysis buffer (30 mM HEPES pH7.4, 90 mM NaCl, 1 mM MgSO4, 187 μM CaC12), and mixed to a desired concentration. 200 μl of SHBG-testosterone mixture was loaded on one side of the dialysis membrane and dialyzed overnight against equal volume of dialysis buffer (2000. The equilibrium was achieved by rotating the dialysis plate overnight at 22° C. 16 hours were determined necessary to achieve an equilibrium. Each concentration/condition was tested in 3 different wells, and each titration was repeated at least 2 times.
[0365] Testosterone concentration was measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) assay that has been certified by the Center for Disease Control and has a sensitivity of 2 ng/dL (Bhasin et al 2011).
[0366] Isothermal calorimetry (ITC) was performed using automated Auto-ITC200 calorimeter (MicroCal, Northampton, Mass.) at Biological calorimetry Facility (Huck Institutes of Life Sciences, University Park, Pa.). SHBG was reconstituted in 30 mM HEPES buffer, pH7.4, to a final concentration of 5 μM. Testosterone standard was prepared in DMSO and diluted in protein buffer to 100 μM in 5% DMSO. DMSO was added to SHBG by weight to match DMSO content in testosterone solution. Samples were degassed prior to loading to the calorimeter. Testosterone was injected into protein solution in 15 equal steps 2 μl each. Total reaction volume was 203 μl. Isothermal titration calorimetry experiment was repeated twice. Heat produced by each injection was measured by the calorimeter. Interval between injections was set at 240 seconds so that the temperature could return to baseline. The heat generated after each injection (after subtracting the heat of dilution of ligand in buffer) was integrated to produce calorimetric isotherm depicting the relation of the total heat generated in the reaction to testosterone-to-SHBG molar ratio.
[0367] Numerical Simulations of Allostery
[0368] Various molecular models of testosterone's binding to SHBG were numerically tested using LabVIEW™ (National Instruments, Austin, Tex.) toolkit (Zakharov et al 2012) (available on the world wide web at code.google.com/p/labview-biochemical-framework/). Parameter estimation for the models was performed as described previously (Zakharov et al 2012). Numerical correction for the equilibrium dialysis was incorporated as a part of every simulation model.
Since some of the models and equations (Sodergard et al 1982, Vermeulen et al 1999, Nanjee and Wheeler 1985) were developed essentially before the confirmation of the 2 binding sites per SHBG dimer (Avvakumov et al 2001) we adjusted SHBG concentration by the factor of 2 for these models.
[0369] The fits of both types of binding profiles and ITC to various models were compared by calculating the residuals and χ2 values for each model. The fits to the model incorporating complex allostery consistently gave the smallest χ2 value and residuals
[0370] Assessment of FT Concentrations in Clinical Trials. FT determined using the dynamic model developed in this study (cFTZBJ) and Vermeulen's equation (cFTV) (as implemented by Mazer, (Mazer 2009)) were compared with those measured using equilibrium dialysis in samples derived from randomized testosterone trials in men (Spitzer et al 2012) and women (Huang et al 2012). These samples had been collected in fasting state in the morning, stored at −80° C., and never thawed.
[0371] Testosterone in Men with Erectile Dysfunction (TED) Trial, whose results have been published (Spitzer et al 2012)(Spitzer et al 2012), was a randomized trial to determine whether addition of testosterone to an optimized regimen of sildenafil citrate is superior to placebo in improving erectile function in men with erectile dysfunction (ED) and low testosterone. At baseline and after 12-weeks of testosterone or placebo administration, total testosterone concentrations were measured using LC-MS/MS and SHBG concentrations using a two-site immunofluorometric assay (DELFIA®, Perkin-Elmer, Waltham, Mass.) (21). FT was measured in the same samples by equilibrium dialysis(Bhasin et al 2012).
[0372] Statistical Analysis.
[0373] The model fits of experimental data were assessed using chi-square statistics. For clinical trials data, the distributions of measured and calculated FT were derived for each of the relevant samples. Agreement between measured and calculated FT values was estimated using Deming (orthogonal) Regression, and Bland-Altman style plots were used to assess the difference between calculated and measured concentrations as a function of the measured concentration. Graphical depictions of association between FT, total testosterone, and SHBG were generated, with scatter plot smoothing using Generalized Additive Models with tensor product smooths (Wood 2006).
[0374] Results
[0375] Preliminary studies revealed that cFT values obtained using the Vermeulen's equation in samples derived from the TED Trial were significantly lower than those measured by equilibrium dialysis. To determine the molecular basis of this discrepancy, three experimental approaches were used to characterize testosterone's binding to SHBG: binding isotherms, ligand depletion curves, and isothermal titration calorimetry (ITC). The overview of different molecular models is presented below. Each of this models assume 2 binding sites per SHBG dimer.
[0376] The simplest of the SHBG T interaction models is Vemeulens model, assuming that each bindingsite interacts with T with the same affinity, regardless of the other binding site occupancy (FIG. 6, model A). The monomers are not interacting, therefore only one subunit is depicted. Second model is a model, when non-interacting monomers are allowed to have different affinities (FIG. 6, model B). Third and fourth models are models of positive and negative cooperativity as postulated by (Koshland et al 1966). Binding of the first testosterone molecule either facilitates (FIG. 6, model C) or supresses (FIG. 6, model D) binding of the second molecule (symmetric reactions are not listed for clarity). The sign of the cooperativity is modelled by the relation of the first equilibrium binding constant (Kd1) to the second one (Kd2). Kd1>Kd2 means positive cooperativity, Kd1<Kd2 means negative cooperativity.
Model in FIG. 6model E, the new Multi-step Dynamic Binding Model with Complex Allostery. The equilibrium between those states while unbound is governed by a unimolecular equilibrium constant Kd11. Upon binding of the first testosterone molecule (with equilibrium constants Kd1 and Kd2) SHBG dimer assumes two different states, each of them with different affinity for the second T molecule (Kd2 and Kd4). Consistent with the reported crystal structure of liganded SHBG (Grishkovskaya et al 2000, Grishkovskaya et al 1999, Grishkovskaya et al 2002, Avvakumov et al 2002), all models were constrained to eventually converge to a single double-liganded conformational state of SHBG dimer. These models were examined and the model with the best fit of the experimental data was determined.
[0377] Biophysical characterization of testosterone's binding to SHBG reveals evidence of complex homo-allostery within SHBG dimer.
[0378] Equilibrium Dialysis: Binding Isotherms
[0379] To generate the binding isotherms, 5, 10 or 20 nM SHBG (dimer) was incubated with graded concentrations of testosterone (0 to 400 nM) at 22° C., as described in the methods section. When bound testosterone concentration was plotted against total testosterone concentration (FIG. 5A, FIG. 8A), the binding isotherm displayed several characteristic features: two distinct saturation plateaus (including an apparent plateau at lower testosterone concentrations), asymmetry of the isotherm around the EC50 value. The relation of bound testosterone to total testosterone could not be adequately explained by Vermeulen's model. Only the new model eliciting intra-dimer allostery fit the binding isotherm optimally with the lowest χ2 (FIG. 8A) and explained the observed saturation plateaus, including the plateau at lower testosterone concentrations, and the asymmetry of binding isotherm around EC50. FIG. 5A presents the fit to the new Multi-step Dynamic Binding Model with Complex Allostery. Fits to other models of FIG. 6 as well as the analysis of the residuals is presented in supplementary material (FIG. 8A-8B). Additionally, it was tested if S*ST and S**S′T microstates were distinguishable. It was found that model with converged S*ST and S**S′T states failed to fit the data.
[0380] Equilibrium Dialysis: Testosterone Depletion Curves
[0381] As an independent assessment of testosterone's binding to SHBG, various amounts of SHBG (0.1 to 0.5 μM) were incubated with a fixed concentration of testosterone, and the depletion of unbound testosterone when increasing concentrations of SHBG were added was analyzed (FIG. 5B, FIGS. 9A-9C). These depletion curves were generated at various testosterone concentrations (3, 6, 8.7, and 16 nM). Sample preparation and measurement procedure are described in the Methods section. The relation of FT to SHBG concentration in depletion experiments was again best fit using the model that included complex allostery. The analysis of residuals (FIG. 8B) revealed that the optimal fit once again provided by the new Multi-step Dynamic Binding Model with Complex Allostery model.
[0382] Isothermal Titration Calorimetry (ITC):
[0383] To validate the new model further and to evaluate the thermodynamic parameters associated with testosterone's binding to SHBG, the heat produced as progressively larger amounts of testosterone bind to SHBG, were measured using the ITC. The ITC data has a characteristic shoulder (FIG. 5C) and cannot be described as a simple sigmoidal curve predicted by Vermeulen's model (FIG. 8A-8B). Using the computational framework developed in LabVIEW (Zakharov et al 2012), we generated the fits of the ITC data (For mathematical treatment, see supplementary material 51, which follows (Freiburger et al 2011)). The shape of ITC curve can be explained as a convoluted result of testosterone's binding and multiple conformational rearrangements defined by the comprehensive model incorporating allostery. Model constants obtained as a result of linked fit in FIGS. 5A and 5B were used as a starting point for the fit; enthalpies and reaction constants computed from the fit ITC data are presented in FIG. 7. While we used an independent enthalpy parameter for each reaction in the model, they are not simultaneously identifiable.
[0384] Effects of Estradiol and Dihydrotestosterone (DHT). Addition of estradiol 17P in concentrations ranging from 10 to 500 pg/mL had no significant effect on percent free testosterone. Similarly, free testosterone concentrations in men treated with graded doses of testosterone enanthate plus placebo whose DHT concentrations extended from physiologic to supraphysiologic range did not differ from those treated with testosterone enanthate plus dutasteride whose DHT concentrations were very low (Bhasin et al 2012), indicating that DHT over the range of concentrations relevant in male and female physiology has little effect on percent free testosterone.
[0385] Application of new Multi-step Dynamic Binding Model with Complex Allostery to Clinical Trials Data. SHBG and albumin are predominantly the two proteins that bind testosterone with significant affinity; the binding affinities of transcortin and orosomucoid for testosterone are extremely low. Accordingly, we included testosterone's interaction with albumin along with complex allostery in equilibria describing its binding to SHBG to determine FT (FTZBJ) in serum samples from the Testosterone in Erectile Dysfunction (TED) Trial ((Spitzer et al 2012). The comprehensive model was implemented in the LabVIEW framework (Zakharov et al 2012) (data not shown).
[0386] cFTV significantly underestimated FT levels relative to equilibrium dialysis in men participating in the TED trial. In contrast, cFTZBJ provided values that were not statistically different from those measured by equilibrium dialysis in men (slope 1.01±0.01). The Bland-Altman plots (data not shown) found no significant difference between the cFTZBJ and those obtained using equilibrium dialysis in either men or women; the relative deviation of values calculated using the new model from those measured using equilibrium dialysis was evenly distributed around 0, likely reflecting multiple sources of measurement error in testosterone assay, SHBG assay, and equilibrium dialysis. The Deming regression was used to compare the values derived using the new model and cFTv with those obtained using equilibrium dialysis, which reaffirm the substantial bias of FTv from values derived using equlibirum dialysis and the substantially better correspondence between cFTZBJ and equilibrium dialysis.
[0387] Relation between Percent FT with Total Testosterone and SHBG. Intra-dimer complex allostery suggests that SHBG can regulate FT fraction over a wide range of total testosterone concentrations without getting saturated. Indeed, it was found that percent FT calculated using the new model changed very modestly over a wide range of total testosterone concentrations. In contrast, the Vermeulen's equation suggests a negative relation between percent FT and total testosterone. Furthermore, as SHBG concentrations increase, percent FT calculated using our new model shows only a modest decline in contrast to the marked decline in percent FT calculated using Vermeulen's equation.
[0387] Discussion
[0389] Several lines of evidence presented here indicate that the existing model of testosterone's binding to SHBG (single binding site or two identical, non-interacting binding sites on SHBG) that has formed the basis of Vermeulen, Sodergard, and Mazer's (Sodergard et al 1982, Vermeulen et al 1971, Mazer 2009, Vermeulen et al 1999) equations to estimate free testosterone concentrations does not accurately explain the experimental data from equilibrium dialysis and and ITC (even if corrected for 2 binding sites per dimer stoichiometry). While the discrepancy between testosterone concentrations estimated using the above-mentioned equations and those measured using equilibrium dialysis has been recognized (Ly et al 2010), the data presented herein provide a mechanistic explanation for this discrepancy. Simple models of homotropic allostery with positive or negative cooperativity within a dimer also did not adequately explain the experimental data. Only the dynamic model that incorporates complex allostery optimally fits the experimental data derived from three independent methods. Furthermore, FT concentrations calculated using the new model incorporating complex allostery were not significantly different from those measured by equilibrium dialysis in samples derived from men and women in two separate clinical trials. The analysis of the steady state experimental binding data presented herein indicate that in the absence of testosterone, SHBG molecule can assume one of at least two inter-converting microstates in dynamic equilibrium. The binding of testosterone to one of the monomers of the SHBG dimer in a given microstate affects the interaction of testosterone with the unoccupied second binding site on the SHBG dimer. The model suggests a dynamic re-adjustment of populations of intermediate species as testosterone concentration is changing. Because of the dynamic nature of these processes, all parameters of the model cannot be uniquely determined Thus, testosterone's binding to SHBG is not a single linear reaction but rather a series of inter-related molecular processes that can be described by the new Multi-step Dynamic Binding Model with Complex Allostery shown in FIG. 7. The fits of the data to the new model that incorporates complex allostery display a dynamic re-adjustment of populations of intermediate species as testosterone concentration changes. Because of the multiple equilibria and dynamic and allosteric nature of these processes, testosterone's binding to SHBG cannot be described as a simple linear equation of ligand binding equilibrium. Accordingly, the multi-species allostery model was implemented in LabVIEW framework (Zakharov et al 2012). Optimal fit parameters for the ITC and equilibrium dialysis data (sections 3.1.1, 3.1.2, 3.1.3) were obtained by the Levenberg-Marquardt optimization, using globalfit approach similar to (Freiburger et al 2011). This set of parameters that can be used to compute free testosterone are shown in FIGS. 7 and 6, model E.
[0390] The new dynamic model leads to the reconsideration of several dogmas related to testosterone's binding to SHBG and has important physiologic and clinical implications. First, the fraction of circulating testosterone which is free is substantially greater (2.9±0.4%) than has been generally assumed (% cFTV 1.5±0.4%). Second, percent FT is not significantly related to total testosterone over a wide range of total testosterone concentrations. However, the percent FT declines as SHBG concentrations increase, although it does not decline as precipitously as predicted by the Vermeulen's model. Due to the allostery between the two binding sites, SHBG is able to regulate FT levels in much larger dynamic range.
[0391] Several factors may have contributed to the formulation of the prevailing hypothesis that monomers within SHBG dimer display identical binding affinity without any dynamic interaction between the monomers. The extant ligand binding equations were formulated in an era that preceded the appreciation of the dimeric nature of circulating SHBG. However, the Mazer's implementation (Mazer 2009) of the Vermeulen's model, as applied in these analyses used the correct stoichiometry—two molecules of testosterone binding to each SHBG dimer Therefore, the discrepancy between cFTv and the reference method cannot be explained solely on the basis of incorrect stoichiometry. Furthermore, the range of testosterone and SHBG concentrations used in binding experiments and Scatchard plots were limited and did not generally extend into the high range (Metzger et al 2003, Dunn et al 1981, Hauptmann et al 2003, Petra et al 1986), which may have prevented appreciation of the second binding site. Also, a single crystal structure of the ligand-bound SHBG may have further contributed to the erroneous impression that the binding events associated with testosterone's binding to two binding sites on SHBG dimer are identical. The inability to resolve the unliganded SHBG structure (Avvakumov et al 2000, Avvakumov et al 2010)(Avvakumov et al 2010) as well as the increased stability of SHBG upon ligand binding (Avvakumov et al 2000)(Avvakumov et al 2000) may be related to significant rearrangement of SHBG molecule upon binding of the first ligand, as predicted by the conformational heterogeneity in complex allostery. The additional energy barrier that SHBG has to overcome may result in altered affinity for binding of the second ligand molecule.
[0392] While the new algorithm developed in this study accurately determines FT, effects of other interacting hormones mandates further investigation. In a previous study (Bhasin et al 2012), it was found that free testosterone concentrations in men treated with graded doses of testosterone enanthate plus placebo whose DHT concentrations extended from physiologic to supraphysiologic range did not differ from those treated with testosterone enanthate plus dutasteride whose DHT concentrations were very low, indicating that DHT over the range of concentrations relevant in in male and female physiology has little effect on percent free testosterone. Similarly, over a wide range of estradiol concentrations prevalent in men and women, cFTZBJ concentrations were similar to those measured using equilibrium dialysis. Without wishing to be bound by theory, very high estrogen concentrations, such as those observed during pregnancy, or very high DHT concentrations could affect testosterone's binding to SHBG.
[0393] Transcortin and orosomucoid display very low affinity for SHBG; their role in regulating free testosterone was not assessed in this investigation and needs further clarity; it is remarkable that FT concentrations derived using the new dynamic model were not significantly different from those determined by equilibrium dialysis in randomized trials even though inter-individual differences in transcortin, and orosomucoid were not considered, consistent with the view that these proteins play a minor role in regulating free testosterone in healthy men and women.
[0394] The current algorithm and the experimental data were generated using wild type SHBG which is present in nearly 98% of Caucasians.
Genome wide association studies have revealed several SHBG polymorphisms, two of which affect testosterone's binding to SHBG (Ohlsson et al 2011)(Ohlsson et al 2011). Therefore, in future, the algorithm may include a term for SHBG genotype. Additional research is needed to extend the model to incorporate SHBG polymorphisms that affect testosterone's binding to SHBG.
[0395] In summary, experimental data generated using several independent methods provide evidence of an complex allostery mechanism of testosterone binding to SHBG dimer FT concentrations derived using the new dynamic model incorporating complex allostery do not differ significantly from those measured using equilibrium dialysis. The application of the new dynamic model to clinical trials data have revealed new insights into the percent of circulating testosterone that is free, the relation between percent FT and total testosterone and SHBG. The use of additional experimental models, including dimerization-deficient SHBG mutants, would allow further characterization of testosterone's interaction with SHBG to validate the complex allostery as suggested by this study.