Kettlebells
Member
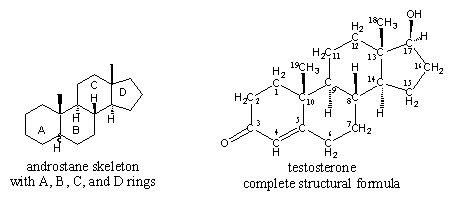
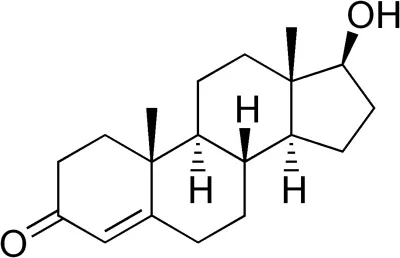
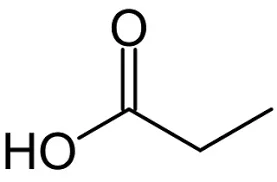
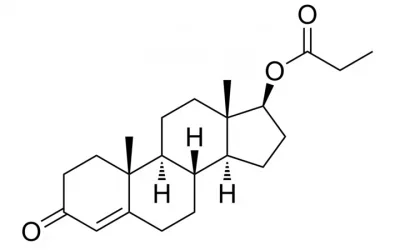
I am assuming that testosterone esters are testosterone bound to an ester. From organic chemistry, we know that an ester is an alcohol bound to a carboxylic acid, which is an organic acid that contains a carboxyl group (i.e., an acid with a chemical formula that ends in COOH). In the case of testosterone cypionate, the carboxylic acid is cypionic acid. Is ethanol the alcohol?