FRI-132: Effects of Continuous Treatment up to 11 Years with Testosterone Undecanoate Injections (TU) in 115 Hypogonadal Men on Hormonal, Anthropometric and
Abstract:Introduction:
The longest-term follow-up on testosterone replacement therapy (TRT) in hypogonadal men in the literature is 6 years. In this registry study, we assessed effects of TRT beyond this period.
Methods:
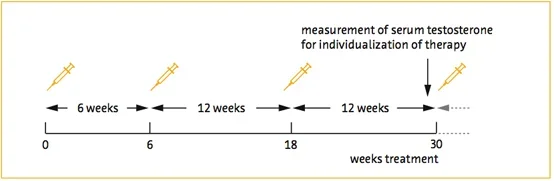
Single-center, cumulative, prospective, registry study of 262 hypogonadal men receiving TU for up to 11 years. Cut-off for total testosterone (T): ≤ 12 nmol/L (~350 ng/dl). In 147 men, TRT had been temporarily discontinued due to reimbursement issues or diagnosis of prostate cancer. In 115 men reported here, TRT was never interrupted for up to 11 years. 115 men were treated for a minimum of 4 years, 114 for 5, 97 for 6, 70 for 7, 57 for 8, 48 for 9, and 23 for 10 years. 4 patients dropped out, 2 due to relocation, 2 were lost to follow-up for unknown reasons. Measures were taken at every other visit. Results at the end of 10 years follow-up are reported.
Results:
Mean age was 59.05 ± 9.36 years (min.: 19; max.: 80).
Total T increased from 7.84 ± 2.34 nmol/L to trough levels (measured prior to the following injection) between 17 and 20 nmol/L, free T from 150.31 ± 65.24 to 400-500 pmol/L, and SHBG decreased from 40.12 ± 20.73 to 33.11 ± 22.14 nmol/L (p<0.0001).
Mean waist circumference decreased from 106.47 ± 8.72 to 92.33 ± 5.32 cm. The decrease was statistically significant vs baseline (p<0.0001) and significant vs previous year for the first 7 years. Mean weight decreased from 97.3 ± 12.88 to 84.65 ± 7.04 kg. The decrease was statistically significant vs baseline (p<0.0001) and significant vs previous year for the first 8 years. Mean BMI decreased from 30.81 ± 4.33 to 27.06 ± 2.49 kg/m[SUP]2[/SUP] The decrease was statistically significant vs baseline (p<0.0001) and significant vs previous year for the first 8 years. Weight reduction was progressive and accumulated to 18.51 ± 6.46% after 10 years. The reduction of waist circumference was 11.98 ± 5.03%.
Total cholesterol (TC) decreased from 251.15 ± 46.77 to 172.02 ± 12.09 mg/dl (p<0.0001 vs baseline, statistical significance vs previous year for the first 7 years), LDL from 156.9 ± 25.43 to 98.09 ± 17.62 (p<0.0001 vs baseline, statistical significance vs previous year for the first 3 years), triglycerides (TG) from 235.72 ± 89.26 to 158.04 ± 20.99 (p<0.0001 vs baseline, statistical significance vs previous year for the first 4 years). HDL increased from 42.41 ± 12.61 to 56.52 ± 6.34 mg/dl (p<0.0001 vs baseline, statistical significance vs previous year for the first 3 years).
TC:HDL ratio improved from 6.59 ± 2.82 to 3.08 ± 0.33 (p<0.0001). Non-HDL cholesterol decreased from 208.74 ± 52.35 to 115.5 ± 11.31 mg/dl (p<0.0001, statistical significance vs previous year for the first 7 years). TG:HDL ratio improved from 6.24 ± 3.53 to 2.82 ± 0.38 (p<0.0001).
No major adverse cardiovascular event (MACE) occurred during the entire observation time.
Conclusions:
Long-term TRT improved anthropometric and lipid parameters in hypogonadal men in a meaningful and sustained fashion.
Disclosure: AAY: Investigator, Bayer Schering Pharma. GD: statistical analyses, Bayer Schering Pharma. FS: Employee, Bayer Schering Pharma. Nothing to Disclose: DJY, AMT
Sources of Research Support: Bayer Pharma AG partially funded data entry and statistical analyses.
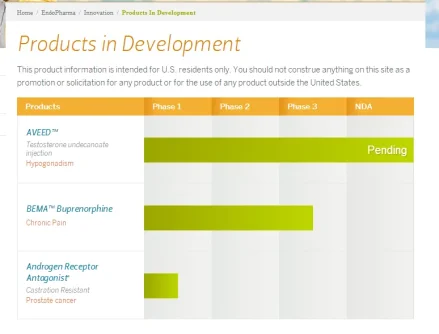
 (NDA) for its long-acting testosterone undecanoate injection, Aveed, intended for men diagnosed with hypogonadism. In connection with the acceptance, the FDA assigned Endo's NDA a new Prescription Drug User Fee Act (PDUFA) action date of Feb. 28, 2014."
(NDA) for its long-acting testosterone undecanoate injection, Aveed, intended for men diagnosed with hypogonadism. In connection with the acceptance, the FDA assigned Endo's NDA a new Prescription Drug User Fee Act (PDUFA) action date of Feb. 28, 2014."