Phil Goodman
Well-Known Member
Wasn’t sure where to put this, so mods feel free to move if needed. Just wanted to share this because I’ve been trying to make more of an effort to minimize my exposure to EDCs lately and came across a searchable database. If you haven’t studied up on EDCs I suggest looking into it, and I’ll probably drop some resources in this thread over time. First link below is the site where you can search by name to see if something in your shampoo, soap, shaving cream, etc. is an EDC. 2nd link is a detailed overview of EDCs.

 www.nature.com
www.nature.com
Endocrine-disrupting chemicals (EDCs) are exogenous chemicals that interfere with hormone action, thereby increasing the risk of adverse health outcomes, including cancer, reproductive impairment, cognitive deficits and obesity. A complex literature of mechanistic studies provides evidence on the hazards of EDC exposure, yet there is no widely accepted systematic method to integrate these data to help identify EDC hazards.
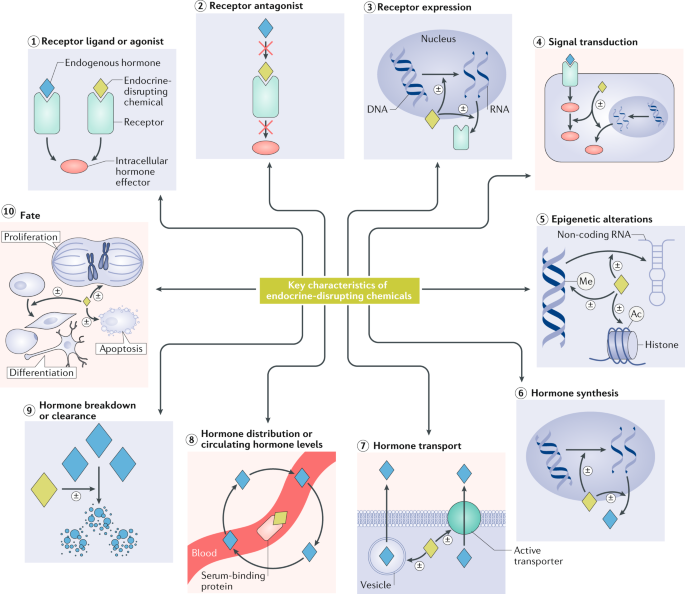
The site also lists 10 key characteristics of EDCs, the first of which are:
KC1: Interacts with or activates hormone receptors
All hormones act by binding to a specific receptor or receptors1. Inappropriate receptor activation can have profound negative effects on development and health, as illustrated by the formation of a scrotum and penis in genetic female humans exposed to androgens during gestation31. EDCs that inappropriately bind to and/or activate hormone receptors can produce adverse biological effects. There are numerous examples of chemicals that cause adverse effects after binding to nuclear hormone receptors. For example, EDCs that inappropriately activate the oestrogen receptors (ERα and ERβ) during development increase the risk of infertility in both sexes as well as reproductive tract cancer in women and prostate cancer in men32, in addition to other reproductive effects. Another example of an EDC that activates hormone receptors is that of dichlorodiphenyltrichloroethane (DDT; Box 1), which binds to ERα and ERβ33 and stimulates ER-dependent transcriptional activation and proliferation34 in a variety of species, including humans. Likewise, a specific hydroxylated congener of a polychlorinated biphenyl (PCB; Box 1) can activate human thyroid hormone receptor-β-mediated transcription1,35. EDCs can also activate cell membrane receptors of peptide and steroid hormones. For instance, DDT binds to the transmembrane domain of the follicle-stimulating hormone receptor, a G protein-coupled receptor (GPCR), to allosterically enhance its stimulation of cAMP production36.
KC2: Antagonizes hormone receptors
EDCs can inhibit or block effects of endogenous hormones by acting as receptor antagonists30. Although antagonism of membrane hormone receptors or intracellular hormone receptors can occur (as exemplified by drug discovery efforts37,38,39), most exogenous chemical research into antagonization of receptors has focused on antagonization of nuclear hormone receptors. Nuclear receptors that act as ligand-dependent transcription factors by mediating genomic regulatory responses can be antagonized by some EDCs. For example, dichlorodiphenyldichloroethylene, an organochlorine pesticide (Box 1), inhibits androgen binding to the androgen receptor (AR) and inhibits androgen-dependent transactivation of the AR in human40 and rat prostrate cells41. Other organochlorine pesticides (such as lindane and dieldrin, which is closely related to the organochlorine insecticide aldrin) also inhibit dihydrotestosterone binding to the AR. As androgens are key regulators of male sexual differentiation during fetal development, disruption of androgen action through AR antagonism in this period can permanently demasculinize male fetuses and lead to malformations of the genital tract42,43.
KC3: Alters hormone receptor expression
As hormone receptors mediate hormone actions1, their physiotemporal pattern of expression dictates their response to hormone signals44,45. For example, receptor abundance can determine both the concentration of hormones that produces an effect as well as the magnitude of the effect itself in some situations46. EDCs can modulate hormone receptor expression, internalization and degradation. For example, di(2‐ethylhexyl) phthalate decreases the expression of the mineralocorticoid (aldosteron) receptor (MR) in the testis of adult mice47, where under normal conditions, MR acts as a positive modulator of testosterone biosynthesis48. Further, BPA (Box 1) alters the expression of oestrogen, oxytocin and vasopressin receptors in brain nuclei49,50,51,52,53, and also reduces the proteasome-mediated degradation of ERβ54. The internalization of cell surface receptors is also disrupted by chemicals. For example, DDT prevents the internalization of the TSH receptor55.
KC4: Alters signal transduction in hormone-responsive cells
The binding of a hormone to a receptor triggers specific intracellular responses that are dependent on the receptor and tissue-specific properties of the target cell. Signal transduction mediated through both membrane and intracellular hormone receptors is altered by some EDCs. The signalling of two classes of receptors will be discussed here as they are the most extensively studied in the field of endocrinology and have EDC effects; these receptors are cell surface membrane receptors (such as GPCRs, receptor kinases, and kinase-linked and ionotropic receptors) and nuclear steroid hormone receptors.
Ionotropic receptor signalling can be perturbed by EDCs. For example, BPA blocks low glucose-induced calcium signalling in isolated pancreatic glucagon-secreting α-cells from adult male mice56. Furthermore, in 2018 it was shown that chemicals in ultraviolet filters disrupt calcium signalling in human sperm57,58.
Some membrane GPCRs bind steroids; among these, G protein-coupled oestrogen receptor (GPER; previously called GPR30) signalling is the best studied regarding the EDC effects (for example, BPA59). Further, EDCs can attenuate or potentiate hormone action through signal transduction. For instance, in in vitro studies, the fungicide tolylfluanid impairs insulin action by reducing insulin receptor substrate 1 (ref.60), while methoxyacetic acid (Box 1) potentiates ligand-activated transcription and progesterone receptor-mediated transcription in a manner dependent on MEK1 and MEK2 activity61.
EDCs also affect signal transduction initiated by nuclear receptors. These effects include their interactions with coregulatory factors such as activators and repressors, which are a key part of the molecular machinery determining the downstream response to nuclear hormone receptor activation. The coregulatory factors for the steroid receptor coactivator (SRC) family are among the most studied in exogenous chemical research. For example, xenoestrogens (such as DES, PCBs, octylphenol and BPA; Box 1) induce the recruitment of SRC1 by ERα and ERβ in a dose-dependent manner62. BPA and its analogues also recruit SRC1 to thyroid hormone receptor-β63. Substantial evidence suggests that xenoestrogens, especially BPA, increase SRC1 expression, as shown in the rat hypothalamus64,65 and in human breast cancer cell lines66. Another EDC, 4-methylbenzylidene camphor (which is used in ultraviolet filters), also increases SRC1 expression in female rat hypothalamus67.


Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification - Nature Reviews Endocrinology
In this Expert Consensus Statement, the authors define 10 key characteristics (KCs) for endocrine-disrupting chemicals. They further describe the logic by which these KCs are identified and the assays that could be used to assess several of these KCs.
Endocrine-disrupting chemicals (EDCs) are exogenous chemicals that interfere with hormone action, thereby increasing the risk of adverse health outcomes, including cancer, reproductive impairment, cognitive deficits and obesity. A complex literature of mechanistic studies provides evidence on the hazards of EDC exposure, yet there is no widely accepted systematic method to integrate these data to help identify EDC hazards.
The site also lists 10 key characteristics of EDCs, the first of which are:
KC1: Interacts with or activates hormone receptors
All hormones act by binding to a specific receptor or receptors1. Inappropriate receptor activation can have profound negative effects on development and health, as illustrated by the formation of a scrotum and penis in genetic female humans exposed to androgens during gestation31. EDCs that inappropriately bind to and/or activate hormone receptors can produce adverse biological effects. There are numerous examples of chemicals that cause adverse effects after binding to nuclear hormone receptors. For example, EDCs that inappropriately activate the oestrogen receptors (ERα and ERβ) during development increase the risk of infertility in both sexes as well as reproductive tract cancer in women and prostate cancer in men32, in addition to other reproductive effects. Another example of an EDC that activates hormone receptors is that of dichlorodiphenyltrichloroethane (DDT; Box 1), which binds to ERα and ERβ33 and stimulates ER-dependent transcriptional activation and proliferation34 in a variety of species, including humans. Likewise, a specific hydroxylated congener of a polychlorinated biphenyl (PCB; Box 1) can activate human thyroid hormone receptor-β-mediated transcription1,35. EDCs can also activate cell membrane receptors of peptide and steroid hormones. For instance, DDT binds to the transmembrane domain of the follicle-stimulating hormone receptor, a G protein-coupled receptor (GPCR), to allosterically enhance its stimulation of cAMP production36.
KC2: Antagonizes hormone receptors
EDCs can inhibit or block effects of endogenous hormones by acting as receptor antagonists30. Although antagonism of membrane hormone receptors or intracellular hormone receptors can occur (as exemplified by drug discovery efforts37,38,39), most exogenous chemical research into antagonization of receptors has focused on antagonization of nuclear hormone receptors. Nuclear receptors that act as ligand-dependent transcription factors by mediating genomic regulatory responses can be antagonized by some EDCs. For example, dichlorodiphenyldichloroethylene, an organochlorine pesticide (Box 1), inhibits androgen binding to the androgen receptor (AR) and inhibits androgen-dependent transactivation of the AR in human40 and rat prostrate cells41. Other organochlorine pesticides (such as lindane and dieldrin, which is closely related to the organochlorine insecticide aldrin) also inhibit dihydrotestosterone binding to the AR. As androgens are key regulators of male sexual differentiation during fetal development, disruption of androgen action through AR antagonism in this period can permanently demasculinize male fetuses and lead to malformations of the genital tract42,43.
KC3: Alters hormone receptor expression
As hormone receptors mediate hormone actions1, their physiotemporal pattern of expression dictates their response to hormone signals44,45. For example, receptor abundance can determine both the concentration of hormones that produces an effect as well as the magnitude of the effect itself in some situations46. EDCs can modulate hormone receptor expression, internalization and degradation. For example, di(2‐ethylhexyl) phthalate decreases the expression of the mineralocorticoid (aldosteron) receptor (MR) in the testis of adult mice47, where under normal conditions, MR acts as a positive modulator of testosterone biosynthesis48. Further, BPA (Box 1) alters the expression of oestrogen, oxytocin and vasopressin receptors in brain nuclei49,50,51,52,53, and also reduces the proteasome-mediated degradation of ERβ54. The internalization of cell surface receptors is also disrupted by chemicals. For example, DDT prevents the internalization of the TSH receptor55.
KC4: Alters signal transduction in hormone-responsive cells
The binding of a hormone to a receptor triggers specific intracellular responses that are dependent on the receptor and tissue-specific properties of the target cell. Signal transduction mediated through both membrane and intracellular hormone receptors is altered by some EDCs. The signalling of two classes of receptors will be discussed here as they are the most extensively studied in the field of endocrinology and have EDC effects; these receptors are cell surface membrane receptors (such as GPCRs, receptor kinases, and kinase-linked and ionotropic receptors) and nuclear steroid hormone receptors.
Ionotropic receptor signalling can be perturbed by EDCs. For example, BPA blocks low glucose-induced calcium signalling in isolated pancreatic glucagon-secreting α-cells from adult male mice56. Furthermore, in 2018 it was shown that chemicals in ultraviolet filters disrupt calcium signalling in human sperm57,58.
Some membrane GPCRs bind steroids; among these, G protein-coupled oestrogen receptor (GPER; previously called GPR30) signalling is the best studied regarding the EDC effects (for example, BPA59). Further, EDCs can attenuate or potentiate hormone action through signal transduction. For instance, in in vitro studies, the fungicide tolylfluanid impairs insulin action by reducing insulin receptor substrate 1 (ref.60), while methoxyacetic acid (Box 1) potentiates ligand-activated transcription and progesterone receptor-mediated transcription in a manner dependent on MEK1 and MEK2 activity61.
EDCs also affect signal transduction initiated by nuclear receptors. These effects include their interactions with coregulatory factors such as activators and repressors, which are a key part of the molecular machinery determining the downstream response to nuclear hormone receptor activation. The coregulatory factors for the steroid receptor coactivator (SRC) family are among the most studied in exogenous chemical research. For example, xenoestrogens (such as DES, PCBs, octylphenol and BPA; Box 1) induce the recruitment of SRC1 by ERα and ERβ in a dose-dependent manner62. BPA and its analogues also recruit SRC1 to thyroid hormone receptor-β63. Substantial evidence suggests that xenoestrogens, especially BPA, increase SRC1 expression, as shown in the rat hypothalamus64,65 and in human breast cancer cell lines66. Another EDC, 4-methylbenzylidene camphor (which is used in ultraviolet filters), also increases SRC1 expression in female rat hypothalamus67.














