Nelson Vergel
Founder, ExcelMale.com
Here is what she writes. Please feel free to add a comment to her blogs. She writes for lawyers to support lawsuits and to get more patients to join them.
******
Seven companies have cornered the market on these testosterone replacement therapies (TRT), raking in billions by advertising directly to consumers and urging physicians to prescribe the medications for off-label use as lifestyle drugs. And millions of prescriptions have now been written for these drugs, which were actually only approved by the FDA to treat one relatively uncommon condition. The result? Burgeoning reports of adverse reactions, including death, which has prompted the FDA to issue a safety announcement and require warning labels. Critics have labeled the Low T marketing push by drug companies as “disease mongering.”

Here are some revealing numbers that show how TRTs penetrated the market and affected consumers:
1 sole medical condition the FDA approved TRT drugs to treat: hypogonadism.
7 Big Pharma companies – AbbVie, Lilly, Endo, Auxilium, GSK, Pfizer and Activis — who cornered the market.
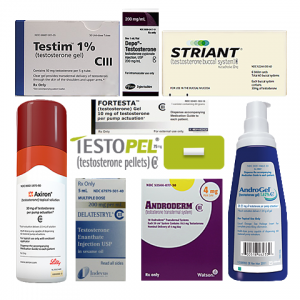
9 heavily marketed TRT drugs: Delatestryl, Testopel, Depo-Testosterone, Androderm, AndroGel, Testim, Striant, Axiron, and Fortesta.
At least 6 different names for the same “condition” that drug companies say the medications can treat: “Low-T,” “Andropause,” Geriatric hypogonadism,” “Male Menopause, ” “Male Climacteric” and “Late-onset male hypogonadism.”
10-question quiz used to self-diagnose whether a consumer thinks he is in need of testosterone drugs. The quiz — which was found on such sites as IsitlowT.com and GetTestedForLowT.com, and is also known as the Androgen Deficiency in Aging Men (or ADAM) questionnaire — was created in 20 minutes in a bathroom by a St. Louis University School of Medicine doctor who scribbled the questions on toilet paper in exchange for a $40,000 grant to the college from Organon BioSciences. Among the questions: “Are you sad and/or grumpy?” (Aren't we all, sometimes?) The quiz still appears on websites.
13 symptoms of Low T advertised by pharmaceutical companies. Symptoms include a decrease in libido, strength, height, endurance, and facial hair and an increase in sadness, fatigue, and sexual dysfunction. Coincidentally — or not — these symptoms are also indicative of aging.
$5 billion in sales of TRT drugs expected by 2017. Sales of testosterone medications rose from $324 million in 2002 to $2 billion in 2012, marking a 517 percent increase
within the decade.
$100 million spent by drug companies in 2012 to market and advertise testosterone products. AbbVie, which sells AndroGel, is one of the biggest spenders, with a marketing budget of $75.6 million in 2012 and $67.9 million in 2013.
$40 million budgeted over a decade by major pharmaceutical companies for Low T and TRT education programs aimed at physicians as part of their efforts to expand the market –or as Axiron called it, “make a bigger pie.” Included in the budgets were at least $2 million in payments to physicians who helped promote the drugs.
20 million men now said to have Low T, according to the manufacturers who sell the drugs. The number increased dramatically within just a few years. In 2002, medical experts estimated the number in just the tens of thousands. But manufacturers expanded the market to one million, then 5 million and then upwards of 20 million.
More than 22,000 adverse events reports filed with FDA regarding Low T medications (Reports can be accessed in sidebar; see infographic here.)
5,000 lawsuits and rising filed against drug companies marketing TRT medication.
[TABLE="width: 800"]
[TR]
[TD]You can read more here:
https://www.truthinadvertising.org/low-down-low-t/
https://www.truthinadvertising.org/low-t-infographic/
https://www.truthinadvertising.org/low-testosterone-timeline/[/TD]
[/TR]
[/TABLE]
******
Seven companies have cornered the market on these testosterone replacement therapies (TRT), raking in billions by advertising directly to consumers and urging physicians to prescribe the medications for off-label use as lifestyle drugs. And millions of prescriptions have now been written for these drugs, which were actually only approved by the FDA to treat one relatively uncommon condition. The result? Burgeoning reports of adverse reactions, including death, which has prompted the FDA to issue a safety announcement and require warning labels. Critics have labeled the Low T marketing push by drug companies as “disease mongering.”
Here are some revealing numbers that show how TRTs penetrated the market and affected consumers:
1 sole medical condition the FDA approved TRT drugs to treat: hypogonadism.
7 Big Pharma companies – AbbVie, Lilly, Endo, Auxilium, GSK, Pfizer and Activis — who cornered the market.
9 heavily marketed TRT drugs: Delatestryl, Testopel, Depo-Testosterone, Androderm, AndroGel, Testim, Striant, Axiron, and Fortesta.
At least 6 different names for the same “condition” that drug companies say the medications can treat: “Low-T,” “Andropause,” Geriatric hypogonadism,” “Male Menopause, ” “Male Climacteric” and “Late-onset male hypogonadism.”
10-question quiz used to self-diagnose whether a consumer thinks he is in need of testosterone drugs. The quiz — which was found on such sites as IsitlowT.com and GetTestedForLowT.com, and is also known as the Androgen Deficiency in Aging Men (or ADAM) questionnaire — was created in 20 minutes in a bathroom by a St. Louis University School of Medicine doctor who scribbled the questions on toilet paper in exchange for a $40,000 grant to the college from Organon BioSciences. Among the questions: “Are you sad and/or grumpy?” (Aren't we all, sometimes?) The quiz still appears on websites.
13 symptoms of Low T advertised by pharmaceutical companies. Symptoms include a decrease in libido, strength, height, endurance, and facial hair and an increase in sadness, fatigue, and sexual dysfunction. Coincidentally — or not — these symptoms are also indicative of aging.
$5 billion in sales of TRT drugs expected by 2017. Sales of testosterone medications rose from $324 million in 2002 to $2 billion in 2012, marking a 517 percent increase
within the decade.

$100 million spent by drug companies in 2012 to market and advertise testosterone products. AbbVie, which sells AndroGel, is one of the biggest spenders, with a marketing budget of $75.6 million in 2012 and $67.9 million in 2013.
$40 million budgeted over a decade by major pharmaceutical companies for Low T and TRT education programs aimed at physicians as part of their efforts to expand the market –or as Axiron called it, “make a bigger pie.” Included in the budgets were at least $2 million in payments to physicians who helped promote the drugs.
20 million men now said to have Low T, according to the manufacturers who sell the drugs. The number increased dramatically within just a few years. In 2002, medical experts estimated the number in just the tens of thousands. But manufacturers expanded the market to one million, then 5 million and then upwards of 20 million.
More than 22,000 adverse events reports filed with FDA regarding Low T medications (Reports can be accessed in sidebar; see infographic here.)
5,000 lawsuits and rising filed against drug companies marketing TRT medication.
[TABLE="width: 800"]
[TR]
[TD]You can read more here:
https://www.truthinadvertising.org/low-down-low-t/
https://www.truthinadvertising.org/low-t-infographic/
https://www.truthinadvertising.org/low-testosterone-timeline/[/TD]
[/TR]
[/TABLE]














