Press release out today. I'd like to see the details of this study, ostensibly a 3-year double blind study using gel, I get the feeling the dosage was likely inadequate.
http://www.brighamandwomens.org/abo...sreleases/PressRelease.aspx?sub=0&PageID=2128
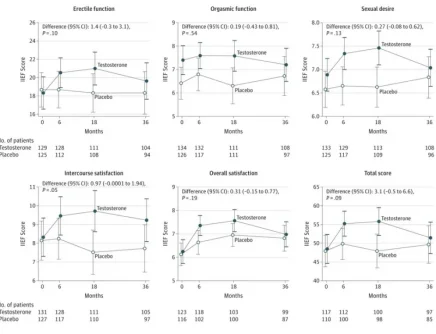
The results of this trial suggest that testosterone should not be used indiscriminately by men,” said corresponding author Shalender Bhasin, MD, director of BWH’s Research Program in Men’s Health: Aging and Metabolism and director of the Boston Claude D. Pepper Older Americans Independence Center at BWH. “We find that men with low and low normal testosterone are unlikely to derive benefits in terms of sexual function or quality of life, two reasons why men may seek testosterone therapy. And although we find that testosterone did not affect the rate of hardening of the arteries, we need long-term data from large trials to determine testosterone’s effects on other major cardiovascular events.”
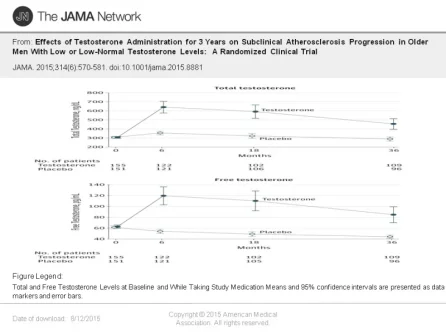
To measure secondary outcomes of sexual function and health-related quality of life, the research team had participants also completed a 15-item questionnaire. Participants applied a testosterone or placebo gel daily for three years.
Testosterone and placebo gel for the study were provided by Solvay Pharmaceuticals, Inc., and later by Abbvie Pharmaceuticals.
http://www.brighamandwomens.org/abo...sreleases/PressRelease.aspx?sub=0&PageID=2128
The results of this trial suggest that testosterone should not be used indiscriminately by men,” said corresponding author Shalender Bhasin, MD, director of BWH’s Research Program in Men’s Health: Aging and Metabolism and director of the Boston Claude D. Pepper Older Americans Independence Center at BWH. “We find that men with low and low normal testosterone are unlikely to derive benefits in terms of sexual function or quality of life, two reasons why men may seek testosterone therapy. And although we find that testosterone did not affect the rate of hardening of the arteries, we need long-term data from large trials to determine testosterone’s effects on other major cardiovascular events.”
To measure secondary outcomes of sexual function and health-related quality of life, the research team had participants also completed a 15-item questionnaire. Participants applied a testosterone or placebo gel daily for three years.
Testosterone and placebo gel for the study were provided by Solvay Pharmaceuticals, Inc., and later by Abbvie Pharmaceuticals.
Last edited by a moderator: