madman
Super Moderator
Most up to date paper!
As usual Nelson house is where its at!
Exogenous T formulations impact on suppression of the HPG axis.
Here is a quick breakdown from most--->least suppressive!
As we have stated numerous times on the forum ultra short-acting Natesto is where its at!
Table 1 | Formulations of testosterone replacement therapy and their effects on male reproductive parameters
Published effects on FSH and LH
LONG-ACTING FORMULATIONS
Nebido/Aveed: Complete or near complete suppression
T-pellets: Strong suppression (90% reduction)
INTERMEDIATE-ACTING FORMULATIONS
T-Cypionate/Enanthate: complete or near complete suppression
Xyosted: No studies identified for the particular product * would be similar to T-Cypionate/Enanthate
SHORT-ACTING FORMULATIONS
Testosterone solution (Axiron): Incomplete suppression (~70% reduction) in men with hypogonadism
T-gels (40.5-50 mg once daily) * keep in mind higher doses would result in stronger suppression of the HPG axis, most men are not even hitting a very high peak TT and more importanly FT on such doses!
Generic: Incomplete suppression (~35% reduction) in men with hypogonadism
Androgel: Incomplete suppression (~40% reduction) in healthy men
Testim: Incomplete suppression (50–70% decrease) in healthy men and men with hypogonadism
Vogelxo: No studies identified for the specific product
Oral testosterone undecanoate (200-237 mg twice daily depending on the formulation)
Tlando: Incomplete suppression in men with hypogonadism
Kyzatrex: Incomplete suppression (~65% reduction) in men with hypogonadism
Jatenzo: Incomplete suppression (~70% reduction) in men with hypogonadism
ULTRA SHORT ACTING FORMULATION (11 mg 3X daily)
Natesto: Incomplete suppression (20–30% decrease) in men with hypogonadism
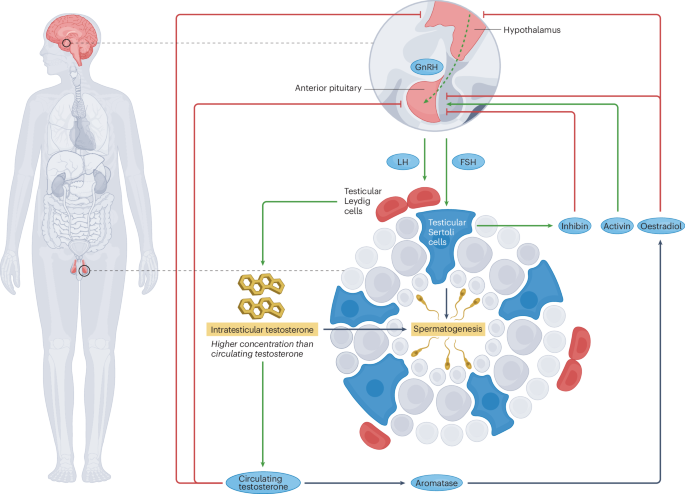
Fig. 2 | Effects of exogenous testosterone on the HPG axis. Exogenous testosterone formulations exert variable inhibitory effects on the hypothalamic– pituitary–gonadal (HPG) axis depending on the properties of the formulation. Long-acting and intermediate-acting testosterone preparations exert near or total suppression of the HPG axis, resulting in a reduced pulsatile release ofgonadotropin-releasing hormone (GnRH; dashed arrow) and blunted releaseof luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to inhibition of testosterone synthesis and spermatogenesis. Prolonged suppression of the HPG axis results in testicular atrophy due to poor stimulation of Leydig and Sertoli cells. Oestradiol and circulating androgen levels rise with administration of exogenous testosterone (especially in the case of anabolic androgenic steroid use), which inhibits the HPG axis at the levels of the hypothalamus and pituitary gland. However, short-acting formulations of exogenous testosterone result in milder inhibition of the HPG axis and might incompletely inhibit intratesticular testosterone synthesis and spermatogenesis.

 www.nature.com
www.nature.com
Abstract
Testosterone has a pivotal role in spermatogenesis, erectile function, libido and expression of secondary sexual characteristics. The prevalence of symptomatic, laboratory-proven testosterone deficiency increases with age and is often treated with testosterone replacement therapy (TRT). Treatment with exogenous androgens suppresses gonadotropin levels, inhibits endogenous testosterone production and drastically reduces intratesticular testosterone, consequently impairing spermatogenesis. Sperm production often slowly resumes after TRT cessation. However, the rate of recovery shows highly variable kinetics that might complicate family planning. Medical therapies (including aromatase inhibitors and selective oestrogen receptor antagonists) and exogenous gonadotropins (including human chorionic gonadotropin and follicle-stimulating hormone) may be used to preserve or restore spermatogenesis in select populations receiving TRT . Exogenous testosterone is contraindicated in men trying to conceive, but new short-acting formulations, including oral testosterone undecanoate and nasal testosterone gel, might incompletely suppress the hypothalamic–pituitary–gonadal axis and partially preserve spermatogenesis.
* Hypothalamic–pituitary–gonadal axis physiology and regulation
* Effects of testosterone on male reproduction
* Definition, epidemiology and symptoms of testosterone deficiency
* The increasing prevalence of exogenous testosterone use
* Effects of exogenous testosterone on HPG axis physiology
All formulations of exogenous testosterone, whether prescribed by a medical provider or used illicitly, inhibit the HPG axis to some degree72 (Fig. 2). Testosterone and its aromatized metabolite oestradiol activate sex-steroid receptors in hypothalamic neurons that release kisspeptin and GnRH, which decrease the frequency of GnRH pulses and blunt gonadotropin release24. Consequently, Leydig-cell activation is decreased, and intratesticular testosterone synthesis declines. Spermatogenesis might be impaired owing to reduced FSH levels and inadequate intratesticular testosterone. Testicular atrophy might also occur as a consequence of inhibited spermatogenesis73. These adverse effects were reported in the HAARLEM study, a prospective observational cohort study including 100 amateur male athletes who planned a cycle of AAS74. Clinic visits occurred before AAS initiation and for 1 year after completion of the AAS cycle, with endocrinological studies and semen analyses obtained during each encounter. Among 63 participants with normal gonadal function who initiated AAS, total motile sperm counts (TMSCs) decreased from an average of 77.2 million at baseline to 13.0 million during the last week of the cycle (P < 0.0001), with an accompanying decrease in testis volume (17.4 versus 13.2 ml, P < 0.0001) and complete inhibition of gonadotropin synthesis (LH 3.0 versus 0.1 U/l, P < 0.0001; FSH 3.9 versus 0.1 U/l, P < 0.0001). Owing to these adverse reproductive effects, patients considering AAS or TRT should be counselled on the possibility of subfertility following exposure to exogenous androgens. Patients should also be informed that recovery of spermatogenesis might take months or even years in some instances54.
Men with testosterone deficiency considering TRT who are interested in fertility preservation should receive assessment by a provider experienced in managing hypogonadism (such as a reproductive urologist or an endocrinologist)75. In this scenario, the primary clinical goal should be maintaining the integrity of the HPG axis to preserve spermatogenesis to some degree. Before initiating treatment, some appropriate measures should be undertaken, including measuring gonadotropin levels, obtaining a semen analysis and carrying out a testicular examination to screen for underlying testicular dysfunction. Men opting for TRT, especially those with subfertility, should be offered sperm cryopreservation before initiating therapy to provide an option for future assisted reproductive success.
The need for effective counselling on the reproductive sequelae of TRT is especially important considering the rising prevalence of direct-to-consumer men’s health clinics. Results from a 2022 systematic review of literature in which these platforms were assessed showed varying levels of adherence to clinical guidelines in these direct-to consumer clinics76. Interestingly, in one study, a ‘secret shopper’ was used to assess how providers on various online platforms adhered to guideline-based recommendations. Although the secret shopper had normal-range testosterone and desired maintenance of fertility, 6 of 7 online platforms were willing to prescribe TRT9. In fact, a majority of direct-to-consumer clinics that offer TRT probably do not use a urologist as a primary provider77. These findings raise substantial concern that direct-to-consumer clinics might not provide adequate counsel to patients before initiating TRT, which might confer adverse reproductive outcomes in the reproductive-aged male.
* Long-acting testosterone replacement therapy substantially inhibits spermatogenesis
Over a dozen testosterone formulations are approved by the FDA for prescription use78 (Table 1). Much of our understanding of these formulations and their effect on spermatogenesis is derived from research on male hormonal contraception based on exogenous testosterone formulations. In many of these studies, the aim was to achieve complete suppression of spermatogenesis, but the physiological effects observed on male reproductive physiology in these studies are informative when considering the clinical applications of these formulations in the treatment of testosterone deficiency.
----------
Long-acting injectable testosterone provides continuous negative inhibition of the HPG axis and strongly suppresses GnRH release, gonadotropin production and spermatogenesis. For example, results from a randomized, placebo-controlled clinical trial in which the effects of monthly testosterone undecanoate injections (500 or 1,000 mg) were assessed in healthy men showed undetectable gonadotropin levels after 4 months of therapy79. Profound suppression of spermatogenesis was observed, with all men but one in the treatment group developing azoospermia. However, the 1,000-mg dose used in this study exceeds the 750-mg dose currently approved by the FDA80. In a single-dose, non-randomized study including men with hypogonadism, controlled-release testosterone pellets (1,200-mg dose) were also shown to induce strong suppression of gonadotropins (~90% reduction) within 3 months of implantation81. However, this dosage greatly exceeds the 150–450-mg doses recommended for use in the USA. Results from a separate clinical trial for hormonal male contraception showed azoospermia or severe oligospermia (<1 × 106/ml) in 72% of healthy men following implantation of testosterone pellets82. These results indicate that long-acting testosterone agents strongly suppress spermatogenesis and should be avoided in men who hope to maintain fertility during treatment for testosterone deficiency.
Similar to long-acting testosterone, intermediate-duration testosterone formulations also result in near-complete suppression of spermatogenesis. Results from an early study of male contraception in which testosterone enanthate was given to 16 healthy individuals showed that an induction regimen (200-mg injections twice weekly for 2 weeks, weekly for 2 weeks and every other week for 4 weeks) markedly suppressed gonadotropins within 8 weeks of treatment, and induced azoospermia or severe oligozoospermia (<100,000 sperm/ml) in all participants83. In the subset of men receiving a maintenance injection every 10–12 days, LH was completely suppressed, with all participants showing either azoospermia or severe oligozoospermia 83.Similarly, results from a prospective study in which testosterone cypionate (100, 250 or 500 mg) was given to healthy individuals complete suppression of gonadotropins and spermatogenesis was observed within 6 weeks84. Thus, men who hope to preserve HPG axis functionality and reliable spermatogenesis during TRT should avoid these intermediate-action formulations as a monotherapy.
* Short-acting testosterone replacement therapy incompletely inhibits spermatogenesis
Short-acting testosterone preparations might mirror physiological changes in testosterone levels more closely than intermediate-acting and long-acting formulations, and, therefore, might incompletely suppress the HPG axis18. However, HPG-axis inhibition will still occur, and cryopreservation should be offered to patients considering TRT. Topical testosterone gels, typically applied once daily, seem to incompletely suppress the HPG axis and spermatogenesis. Results from a randomized, placebo-controlled trial including men with hypogonadism showed that daily application of transdermal testosterone (50 mg) suppressed LH from 5.1 to 3.3 mIU/ml and FSH from 10.3 to 6.4 mIU/ml after 3 months of use85. However, gonadotropin levels remained within normal limits, suggesting that HPG-axis integrity was maintained even in the presence of exogenous testosterone. Importantly, patients reported improved sexual function based on International Index of Erectile Function (IIEF) scores85. In a pilot study including 18 eugonadotropic men with androgen deficiency, treatment with transdermal testosterone (50 mg daily) for 3 months preserved sperm concentration compared with baseline (23.0 versus 19.0 × 106/ml,P = 0.24) and motility (42.0 versus 42.6%, P = 0.69). These patients experienced a modest but significant rise in serum testosterone (from 8.4 to 16.25 nmol/l, P < 0.001) during treatment, and all patients showed normalized sexual function and improved weight86. In another study including men with severe oligozoospermia, transdermal testosterone (25 mg daily) modestly reduced FSH levels (3.6 mIU/ml months versus 4.8 mIU/ml at baseline, P < 0.0001), preserved LH levels (3.0 versus 3.3 mIU/ml, P = 0.74), and increased testosterone (302 versus 233 ng/dl, P < 0.001) while preserving sperm concentrations (4.0 versus 5.5 × 106/ml, P = 0.19)87. Some patients experienced improved libido and sleep quality. However, this study was ultimately discontinued after a patient experienced an adverse event87. Additionally, the application of topical testosterone poses some risk to the female partner, considering the possibility of transference 88. Nevertheless, short-acting TRT seems to provide relief of symptoms of testosterone deficiency while maintaining integrity of the HPG axis.
Short-acting intranasal testosterone also seems to maintain HPG-axis integrity and permit continued spermatogenesis while transiently increasing testosterone levels89. In a randomized, open-label, multi-centre trial, the efficacy and safety of nasal testosterone gel( 22 mg or 33 mg) were evaluated in men with hypogonadism. After 90 days, 73% of patients in the intent-to-treat population and 76% in the per-protocol population achieved normal-range testosterone levels. Additionally, mood, body composition, bone mineral density and erectile function (based on IIEF scores) improved from baseline 90. In a follow-up open-label, single-arm trial, the effect of nasal testosterone on gonadotropin levels and spermatogenesis was evaluated in 60 men with symptomatic testosterone deficiency with baseline TMSC >5 million who used the agent for 6 months91. By the end of the trial, 82% and 73% of patients maintained FSH and LH levels within normal limits, respectively91. After 3 months of therapy, 88% of men sustained TMSCs ≥5 million with no statistically significant changes reported (40.8 M at baseline versus 25.9 M post-treatment, P > 0.05), which was accompanied by significant improvements in IIEF-15 scores (23.1 versus 25.6, P = 0.005, maximum score 30) and overall satisfaction with sexual function (6.3 versus 7.8, P < 0.001, maximum score 10). The overwhelming majority of participants in this study maintained spermatogenesis above this threshold, although one patient developed azoospermia after 3 months of therapy and three others showed severe oligozoospermia91. Nasal testosterone is not devoid of risks (including sinusitis, nasal irritation and epistaxis), but this treatment does offer promise for patients with primary testosterone deficiency who also desire modest symptomatic improvement and maintenance of fertility. The updated AUA/American Society for Reproductive Medicine guidelines on male infertility acknowledge that short-acting nasal testosterone generally preserved spermatogenesis in these studies91,92,but note that long-term data on reproductive outcomes are lacking and that routine use of this agent cannot be recommended in men trying to conceive89. Furthermore, this medication is dosed three times daily; thus, compliance in the general population is likely to be poor, which might limit the desirability and efficacy of this treatment.
Novel short-acting oral preparations of testosterone undecanoate offer convenience and the potential for increased adherence. Oral testosterone undecanoate has been available in Europe since the late1970s but was repeatedly rejected by the FDA owing to concerns about safety and variable absorption kinetics93. By 2022, however, the FDA approved three preparations of testosterone undecanoate in which the androgen is suspended in a self-emulsifying drug delivery system that results in peak levels 4–6 h after intake93,94. Testosterone undecanoates typically dosed twice daily, as testosterone levels return to baseline levels ~8–12 h after achieving peak concentration95
Considering this short half-life, inhibition of the HPG axis is likely weaker than that achieved with longer-acting testosterone formulations. This effect was shown in an open-label, single-arm, multicentre study including 95 men with androgen insufficiency receiving oral testosterone undecanoate (225 mg twice daily) 96. After 24 days of therapy, LH and FSH declined by a mean of 4.7 mIU/ml and 4.9 mIU/ml, respectively with 40% of participants maintaining normal LH or FSH levels. In another single-arm, multicentre trial including 155 men with hypogonadism receiving a different preparation of oral testosterone undecanoate (200 mg twice daily) 97, LH decreased from 5.9 mIU/ml at baseline to 2.4 mIU/ml, and FSH decreased from 5.8 mIU/ml at baseline to 2.5 mIU/ml after 6 months of use. These modest reductions in LH and FSH reflect an incomplete suppression of the HPG axis, which suggests that spermatogenesis might be maintained during oral testosterone undecanoate therapy. Most available literature does not provide information on semen parameters following oral testosterone undecanoate, but in a very small prospective pilot study, endocrine and semen parameters were assessed in 5 hypogonadal men after 3 months of treatment. In this study, one patient experienced azoospermia, whereas the remaining four patients did not show statistically significant differences in sperm count (P = 0.53), motility (P = 0.10)or volume (P = 0.66) after 3 months98. These results are interesting, but this small study has an inadequate sample size to draw definitive conclusions. Further studies are needed to evaluate whether fertility can be maintained on self-emulsifying oral testosterone undecanoate. Oral formulations cannot yet be recommended as a fertility-sparingapproach owing to the lack of clear evidence showing preservation of spermatogenesis. Furthermore, current AUA guidelines on testosterone deficiency recommend that TRT of any kind should not be prescribed to men trying to conceive54.
* Spontaneous recovery of fertility after cessation of exogenous testosterone
* Medical therapy supports HPG axis recovery and spermatogenesis in men with exogenous testosterone exposure
* Management of testosterone deficiency in reproductive-aged men trying to conceive
* Gamete acquisition and assisted reproductive technology
Conclusions
TRT has never been so widely advertised, available and, unfortunately, misused or abused. Reproductive-aged men are increasingly seeking out testosterone therapies, often without awareness that exogenous testosterone therapy might impair fertility. Most men without baseline testicular dysfunction will spontaneously recover fertility, but months or years of waiting might be required to achieve satisfactory sperm production. For men of reproductive age, appropriate treatment of testosterone deficiency should prioritize maintaining HPG-axis functionality. Interestingly, short-acting testosterone therapies (including topical testosterone gel, nasal testosterone gel and novel oral testosterone undecanoate) seem to incompletely suppress the HPG axis. Men might be able to maintain spermatogenesis while receiving some short-acting testosterone preparations (or recover more quickly after stopping than men receiving longer-acting formulations of testosterone), although additional investigation is necessary to confirm this hypothesis. According to current knowledge, TRT should be avoided in men with testosterone deficiency trying to conceive. Men symptomatic from testosterone deficiency desiring fertility should be prescribed medical therapies that support the HPG axis, stimulate androgenesis and enhance spermatogenesis. Men experiencing infertility secondary to exogenous testosterone might benefit from medical therapy that reconstitutes the HPG axis to help achieve paternity naturally or with the aid of ART. Reproductive urologists not only guide patients through the complexities of fertility restoration but also have a responsibility to educate the public on the reproductive sequelae following TRT.
As usual Nelson house is where its at!
Exogenous T formulations impact on suppression of the HPG axis.
Here is a quick breakdown from most--->least suppressive!
As we have stated numerous times on the forum ultra short-acting Natesto is where its at!
Table 1 | Formulations of testosterone replacement therapy and their effects on male reproductive parameters
Published effects on FSH and LH
LONG-ACTING FORMULATIONS
Nebido/Aveed: Complete or near complete suppression
T-pellets: Strong suppression (90% reduction)
INTERMEDIATE-ACTING FORMULATIONS
T-Cypionate/Enanthate: complete or near complete suppression
Xyosted: No studies identified for the particular product * would be similar to T-Cypionate/Enanthate
SHORT-ACTING FORMULATIONS
Testosterone solution (Axiron): Incomplete suppression (~70% reduction) in men with hypogonadism
T-gels (40.5-50 mg once daily) * keep in mind higher doses would result in stronger suppression of the HPG axis, most men are not even hitting a very high peak TT and more importanly FT on such doses!
Generic: Incomplete suppression (~35% reduction) in men with hypogonadism
Androgel: Incomplete suppression (~40% reduction) in healthy men
Testim: Incomplete suppression (50–70% decrease) in healthy men and men with hypogonadism
Vogelxo: No studies identified for the specific product
Oral testosterone undecanoate (200-237 mg twice daily depending on the formulation)
Tlando: Incomplete suppression in men with hypogonadism
Kyzatrex: Incomplete suppression (~65% reduction) in men with hypogonadism
Jatenzo: Incomplete suppression (~70% reduction) in men with hypogonadism
ULTRA SHORT ACTING FORMULATION (11 mg 3X daily)
Natesto: Incomplete suppression (20–30% decrease) in men with hypogonadism
Fig. 2 | Effects of exogenous testosterone on the HPG axis. Exogenous testosterone formulations exert variable inhibitory effects on the hypothalamic– pituitary–gonadal (HPG) axis depending on the properties of the formulation. Long-acting and intermediate-acting testosterone preparations exert near or total suppression of the HPG axis, resulting in a reduced pulsatile release ofgonadotropin-releasing hormone (GnRH; dashed arrow) and blunted releaseof luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to inhibition of testosterone synthesis and spermatogenesis. Prolonged suppression of the HPG axis results in testicular atrophy due to poor stimulation of Leydig and Sertoli cells. Oestradiol and circulating androgen levels rise with administration of exogenous testosterone (especially in the case of anabolic androgenic steroid use), which inhibits the HPG axis at the levels of the hypothalamus and pituitary gland. However, short-acting formulations of exogenous testosterone result in milder inhibition of the HPG axis and might incompletely inhibit intratesticular testosterone synthesis and spermatogenesis.

Testosterone replacement therapy and spermatogenesis in reproductive age men - Nature Reviews Urology
Testosterone replacement therapy is commonly used to treat symptomatic, laboratory-proven testosterone deficiency, but can have detrimental effects on spermatogenesis, which is troublesome in men of reproductive age. This Review describes therapeutic options for testosterone deficiency in the...
Abstract
Testosterone has a pivotal role in spermatogenesis, erectile function, libido and expression of secondary sexual characteristics. The prevalence of symptomatic, laboratory-proven testosterone deficiency increases with age and is often treated with testosterone replacement therapy (TRT). Treatment with exogenous androgens suppresses gonadotropin levels, inhibits endogenous testosterone production and drastically reduces intratesticular testosterone, consequently impairing spermatogenesis. Sperm production often slowly resumes after TRT cessation. However, the rate of recovery shows highly variable kinetics that might complicate family planning. Medical therapies (including aromatase inhibitors and selective oestrogen receptor antagonists) and exogenous gonadotropins (including human chorionic gonadotropin and follicle-stimulating hormone) may be used to preserve or restore spermatogenesis in select populations receiving TRT . Exogenous testosterone is contraindicated in men trying to conceive, but new short-acting formulations, including oral testosterone undecanoate and nasal testosterone gel, might incompletely suppress the hypothalamic–pituitary–gonadal axis and partially preserve spermatogenesis.
* Hypothalamic–pituitary–gonadal axis physiology and regulation
* Effects of testosterone on male reproduction
* Definition, epidemiology and symptoms of testosterone deficiency
* The increasing prevalence of exogenous testosterone use
* Effects of exogenous testosterone on HPG axis physiology
All formulations of exogenous testosterone, whether prescribed by a medical provider or used illicitly, inhibit the HPG axis to some degree72 (Fig. 2). Testosterone and its aromatized metabolite oestradiol activate sex-steroid receptors in hypothalamic neurons that release kisspeptin and GnRH, which decrease the frequency of GnRH pulses and blunt gonadotropin release24. Consequently, Leydig-cell activation is decreased, and intratesticular testosterone synthesis declines. Spermatogenesis might be impaired owing to reduced FSH levels and inadequate intratesticular testosterone. Testicular atrophy might also occur as a consequence of inhibited spermatogenesis73. These adverse effects were reported in the HAARLEM study, a prospective observational cohort study including 100 amateur male athletes who planned a cycle of AAS74. Clinic visits occurred before AAS initiation and for 1 year after completion of the AAS cycle, with endocrinological studies and semen analyses obtained during each encounter. Among 63 participants with normal gonadal function who initiated AAS, total motile sperm counts (TMSCs) decreased from an average of 77.2 million at baseline to 13.0 million during the last week of the cycle (P < 0.0001), with an accompanying decrease in testis volume (17.4 versus 13.2 ml, P < 0.0001) and complete inhibition of gonadotropin synthesis (LH 3.0 versus 0.1 U/l, P < 0.0001; FSH 3.9 versus 0.1 U/l, P < 0.0001). Owing to these adverse reproductive effects, patients considering AAS or TRT should be counselled on the possibility of subfertility following exposure to exogenous androgens. Patients should also be informed that recovery of spermatogenesis might take months or even years in some instances54.
Men with testosterone deficiency considering TRT who are interested in fertility preservation should receive assessment by a provider experienced in managing hypogonadism (such as a reproductive urologist or an endocrinologist)75. In this scenario, the primary clinical goal should be maintaining the integrity of the HPG axis to preserve spermatogenesis to some degree. Before initiating treatment, some appropriate measures should be undertaken, including measuring gonadotropin levels, obtaining a semen analysis and carrying out a testicular examination to screen for underlying testicular dysfunction. Men opting for TRT, especially those with subfertility, should be offered sperm cryopreservation before initiating therapy to provide an option for future assisted reproductive success.
The need for effective counselling on the reproductive sequelae of TRT is especially important considering the rising prevalence of direct-to-consumer men’s health clinics. Results from a 2022 systematic review of literature in which these platforms were assessed showed varying levels of adherence to clinical guidelines in these direct-to consumer clinics76. Interestingly, in one study, a ‘secret shopper’ was used to assess how providers on various online platforms adhered to guideline-based recommendations. Although the secret shopper had normal-range testosterone and desired maintenance of fertility, 6 of 7 online platforms were willing to prescribe TRT9. In fact, a majority of direct-to-consumer clinics that offer TRT probably do not use a urologist as a primary provider77. These findings raise substantial concern that direct-to-consumer clinics might not provide adequate counsel to patients before initiating TRT, which might confer adverse reproductive outcomes in the reproductive-aged male.
* Long-acting testosterone replacement therapy substantially inhibits spermatogenesis
Over a dozen testosterone formulations are approved by the FDA for prescription use78 (Table 1). Much of our understanding of these formulations and their effect on spermatogenesis is derived from research on male hormonal contraception based on exogenous testosterone formulations. In many of these studies, the aim was to achieve complete suppression of spermatogenesis, but the physiological effects observed on male reproductive physiology in these studies are informative when considering the clinical applications of these formulations in the treatment of testosterone deficiency.
----------
Long-acting injectable testosterone provides continuous negative inhibition of the HPG axis and strongly suppresses GnRH release, gonadotropin production and spermatogenesis. For example, results from a randomized, placebo-controlled clinical trial in which the effects of monthly testosterone undecanoate injections (500 or 1,000 mg) were assessed in healthy men showed undetectable gonadotropin levels after 4 months of therapy79. Profound suppression of spermatogenesis was observed, with all men but one in the treatment group developing azoospermia. However, the 1,000-mg dose used in this study exceeds the 750-mg dose currently approved by the FDA80. In a single-dose, non-randomized study including men with hypogonadism, controlled-release testosterone pellets (1,200-mg dose) were also shown to induce strong suppression of gonadotropins (~90% reduction) within 3 months of implantation81. However, this dosage greatly exceeds the 150–450-mg doses recommended for use in the USA. Results from a separate clinical trial for hormonal male contraception showed azoospermia or severe oligospermia (<1 × 106/ml) in 72% of healthy men following implantation of testosterone pellets82. These results indicate that long-acting testosterone agents strongly suppress spermatogenesis and should be avoided in men who hope to maintain fertility during treatment for testosterone deficiency.
Similar to long-acting testosterone, intermediate-duration testosterone formulations also result in near-complete suppression of spermatogenesis. Results from an early study of male contraception in which testosterone enanthate was given to 16 healthy individuals showed that an induction regimen (200-mg injections twice weekly for 2 weeks, weekly for 2 weeks and every other week for 4 weeks) markedly suppressed gonadotropins within 8 weeks of treatment, and induced azoospermia or severe oligozoospermia (<100,000 sperm/ml) in all participants83. In the subset of men receiving a maintenance injection every 10–12 days, LH was completely suppressed, with all participants showing either azoospermia or severe oligozoospermia 83.Similarly, results from a prospective study in which testosterone cypionate (100, 250 or 500 mg) was given to healthy individuals complete suppression of gonadotropins and spermatogenesis was observed within 6 weeks84. Thus, men who hope to preserve HPG axis functionality and reliable spermatogenesis during TRT should avoid these intermediate-action formulations as a monotherapy.
* Short-acting testosterone replacement therapy incompletely inhibits spermatogenesis
Short-acting testosterone preparations might mirror physiological changes in testosterone levels more closely than intermediate-acting and long-acting formulations, and, therefore, might incompletely suppress the HPG axis18. However, HPG-axis inhibition will still occur, and cryopreservation should be offered to patients considering TRT. Topical testosterone gels, typically applied once daily, seem to incompletely suppress the HPG axis and spermatogenesis. Results from a randomized, placebo-controlled trial including men with hypogonadism showed that daily application of transdermal testosterone (50 mg) suppressed LH from 5.1 to 3.3 mIU/ml and FSH from 10.3 to 6.4 mIU/ml after 3 months of use85. However, gonadotropin levels remained within normal limits, suggesting that HPG-axis integrity was maintained even in the presence of exogenous testosterone. Importantly, patients reported improved sexual function based on International Index of Erectile Function (IIEF) scores85. In a pilot study including 18 eugonadotropic men with androgen deficiency, treatment with transdermal testosterone (50 mg daily) for 3 months preserved sperm concentration compared with baseline (23.0 versus 19.0 × 106/ml,P = 0.24) and motility (42.0 versus 42.6%, P = 0.69). These patients experienced a modest but significant rise in serum testosterone (from 8.4 to 16.25 nmol/l, P < 0.001) during treatment, and all patients showed normalized sexual function and improved weight86. In another study including men with severe oligozoospermia, transdermal testosterone (25 mg daily) modestly reduced FSH levels (3.6 mIU/ml months versus 4.8 mIU/ml at baseline, P < 0.0001), preserved LH levels (3.0 versus 3.3 mIU/ml, P = 0.74), and increased testosterone (302 versus 233 ng/dl, P < 0.001) while preserving sperm concentrations (4.0 versus 5.5 × 106/ml, P = 0.19)87. Some patients experienced improved libido and sleep quality. However, this study was ultimately discontinued after a patient experienced an adverse event87. Additionally, the application of topical testosterone poses some risk to the female partner, considering the possibility of transference 88. Nevertheless, short-acting TRT seems to provide relief of symptoms of testosterone deficiency while maintaining integrity of the HPG axis.
Short-acting intranasal testosterone also seems to maintain HPG-axis integrity and permit continued spermatogenesis while transiently increasing testosterone levels89. In a randomized, open-label, multi-centre trial, the efficacy and safety of nasal testosterone gel( 22 mg or 33 mg) were evaluated in men with hypogonadism. After 90 days, 73% of patients in the intent-to-treat population and 76% in the per-protocol population achieved normal-range testosterone levels. Additionally, mood, body composition, bone mineral density and erectile function (based on IIEF scores) improved from baseline 90. In a follow-up open-label, single-arm trial, the effect of nasal testosterone on gonadotropin levels and spermatogenesis was evaluated in 60 men with symptomatic testosterone deficiency with baseline TMSC >5 million who used the agent for 6 months91. By the end of the trial, 82% and 73% of patients maintained FSH and LH levels within normal limits, respectively91. After 3 months of therapy, 88% of men sustained TMSCs ≥5 million with no statistically significant changes reported (40.8 M at baseline versus 25.9 M post-treatment, P > 0.05), which was accompanied by significant improvements in IIEF-15 scores (23.1 versus 25.6, P = 0.005, maximum score 30) and overall satisfaction with sexual function (6.3 versus 7.8, P < 0.001, maximum score 10). The overwhelming majority of participants in this study maintained spermatogenesis above this threshold, although one patient developed azoospermia after 3 months of therapy and three others showed severe oligozoospermia91. Nasal testosterone is not devoid of risks (including sinusitis, nasal irritation and epistaxis), but this treatment does offer promise for patients with primary testosterone deficiency who also desire modest symptomatic improvement and maintenance of fertility. The updated AUA/American Society for Reproductive Medicine guidelines on male infertility acknowledge that short-acting nasal testosterone generally preserved spermatogenesis in these studies91,92,but note that long-term data on reproductive outcomes are lacking and that routine use of this agent cannot be recommended in men trying to conceive89. Furthermore, this medication is dosed three times daily; thus, compliance in the general population is likely to be poor, which might limit the desirability and efficacy of this treatment.
Novel short-acting oral preparations of testosterone undecanoate offer convenience and the potential for increased adherence. Oral testosterone undecanoate has been available in Europe since the late1970s but was repeatedly rejected by the FDA owing to concerns about safety and variable absorption kinetics93. By 2022, however, the FDA approved three preparations of testosterone undecanoate in which the androgen is suspended in a self-emulsifying drug delivery system that results in peak levels 4–6 h after intake93,94. Testosterone undecanoates typically dosed twice daily, as testosterone levels return to baseline levels ~8–12 h after achieving peak concentration95
Considering this short half-life, inhibition of the HPG axis is likely weaker than that achieved with longer-acting testosterone formulations. This effect was shown in an open-label, single-arm, multicentre study including 95 men with androgen insufficiency receiving oral testosterone undecanoate (225 mg twice daily) 96. After 24 days of therapy, LH and FSH declined by a mean of 4.7 mIU/ml and 4.9 mIU/ml, respectively with 40% of participants maintaining normal LH or FSH levels. In another single-arm, multicentre trial including 155 men with hypogonadism receiving a different preparation of oral testosterone undecanoate (200 mg twice daily) 97, LH decreased from 5.9 mIU/ml at baseline to 2.4 mIU/ml, and FSH decreased from 5.8 mIU/ml at baseline to 2.5 mIU/ml after 6 months of use. These modest reductions in LH and FSH reflect an incomplete suppression of the HPG axis, which suggests that spermatogenesis might be maintained during oral testosterone undecanoate therapy. Most available literature does not provide information on semen parameters following oral testosterone undecanoate, but in a very small prospective pilot study, endocrine and semen parameters were assessed in 5 hypogonadal men after 3 months of treatment. In this study, one patient experienced azoospermia, whereas the remaining four patients did not show statistically significant differences in sperm count (P = 0.53), motility (P = 0.10)or volume (P = 0.66) after 3 months98. These results are interesting, but this small study has an inadequate sample size to draw definitive conclusions. Further studies are needed to evaluate whether fertility can be maintained on self-emulsifying oral testosterone undecanoate. Oral formulations cannot yet be recommended as a fertility-sparingapproach owing to the lack of clear evidence showing preservation of spermatogenesis. Furthermore, current AUA guidelines on testosterone deficiency recommend that TRT of any kind should not be prescribed to men trying to conceive54.
* Spontaneous recovery of fertility after cessation of exogenous testosterone
* Medical therapy supports HPG axis recovery and spermatogenesis in men with exogenous testosterone exposure
* Management of testosterone deficiency in reproductive-aged men trying to conceive
* Gamete acquisition and assisted reproductive technology
Conclusions
TRT has never been so widely advertised, available and, unfortunately, misused or abused. Reproductive-aged men are increasingly seeking out testosterone therapies, often without awareness that exogenous testosterone therapy might impair fertility. Most men without baseline testicular dysfunction will spontaneously recover fertility, but months or years of waiting might be required to achieve satisfactory sperm production. For men of reproductive age, appropriate treatment of testosterone deficiency should prioritize maintaining HPG-axis functionality. Interestingly, short-acting testosterone therapies (including topical testosterone gel, nasal testosterone gel and novel oral testosterone undecanoate) seem to incompletely suppress the HPG axis. Men might be able to maintain spermatogenesis while receiving some short-acting testosterone preparations (or recover more quickly after stopping than men receiving longer-acting formulations of testosterone), although additional investigation is necessary to confirm this hypothesis. According to current knowledge, TRT should be avoided in men with testosterone deficiency trying to conceive. Men symptomatic from testosterone deficiency desiring fertility should be prescribed medical therapies that support the HPG axis, stimulate androgenesis and enhance spermatogenesis. Men experiencing infertility secondary to exogenous testosterone might benefit from medical therapy that reconstitutes the HPG axis to help achieve paternity naturally or with the aid of ART. Reproductive urologists not only guide patients through the complexities of fertility restoration but also have a responsibility to educate the public on the reproductive sequelae following TRT.












