Abstract
IntroductionClomiphene is often used in the treatment of hypogonadal men who wish to preserve endogenous testosterone production, however symptomatic responses are often suboptimal. Exogenous testosterone replacement, while effective, typically suppress the hypothalamic–pituitary-gonadal (HPG) axis. This case series investigates the efficacy of combining clomiphene with high-dose oral testosterone undecanoate (TU) (Kyzatrex, Marius Pharmaceuticals).
Objective
To report the outcomes in a series of patients treated with combined clomiphene and oral TU therapy.
Methods
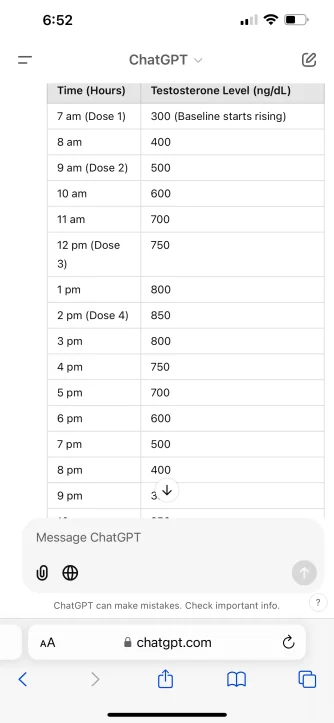
Three hypogonadal men who initially received clomiphene therapy (50 mg every other day) but did not achieve satisfactory symptomatic improvement despite numerical improvement were identified. Subsequently, they were given oral TU 400 mg once daily with lunch. Subjective symptomatic outcomes, total testosterone (TT), sex hormone binding globulin (SHBG), calculated free testosterone (fT), estradiol, hematocrit (Hct), and HPG axis markers luteinizing hormone (LH) and follicle stimulating hormone (FSH) were monitored at baseline, after 3 months of clomiphene monotherapy, and after 3 additional months of combined clomiphene and oral TU therapy.
Results
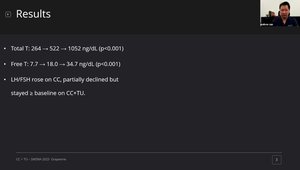
The patients had baseline serum testosterone levels of 303, 223, and 230 ng/dL. After 3 months of clomiphene 50 mg QoD, TT rose to 418, 680, and 667 ng/dL respectively, however these patients reported continued symptoms of hypogonadism. After adding 400 mg oral TU once daily for 3 months, TT rose to 1001, 1055, and 1120 ng/dL, and the patients reported significant improvement in symptoms such as erectile dysfunction, fatigue, libido, and exercise tolerance. Both SHBG and Estradiol levels rose with clomiphene monotherapy but subsequently decreased after adding oral TU. Hct was unchanged throughout. FSH and LH levels both rose on clomiphene and subsequently dropped after initiation of oral TU, but remained near baseline, indicating preserved HPG axis function. No significant adverse events were reported.
Conclusions
In these patients for whom clomiphene monotherapy failed to generate a sufficient symptomatic response, the addition of high-dose oral TU therapy (Kyzatrex 400 mg) at a once-daily dosing resulted in substantial symptomatic improvement while maintaining endogenous FSH and LH levels at baseline. These findings suggest a novel approach to hypogonadism management, balancing effective symptom relief with preservation of endogenous testosterone production, with potential downstream ramifications on fertility preservation.
Last edited: