madman
Super Moderator
PRINCIPLE
INTENDED USE
The Access Sensitive Estradiol assay is a paramagnetic particle, chemiluminescent immunoassay for the quantitative determination of estradiol levels in human serum and plasma using the Access Immunoassay Systems.
SUMMARY AND EXPLANATION
Estradiol (17β-estradiol) is a natural estrogen with a molecular mass of 272.4 daltons. Most circulating estradiol is strongly bound to sex hormone binding protein and loosely bound to albumin. It is estimated that only 1-5% of estradiol is free (unbound). In non-pregnant women,estradiol is secreted by the ovary and the corpus luteum. The adrenals and testes (in men) are also believed to secrete minute amounts of estradiol.
Estradiol levels are lowest at menses and into the early follicular phase and rise in the late follicular phase to a peak just prior to the hLH (human Luteinizing Hormone) surge, initiating ovulation. As the hLH peaks, the levels of estradiol decrease before rising again in the luteal phase. Endometrial growth is stimulated by estradiol and progesterone (secreted by the corpus luteum) in preparation for implantation of a fertilized egg. If conception does not occur, the secretion of estradiol and progesterone by the corpus luteum decreases, initiating menses.
Levels of estradiol are used to monitor ovulatory status. Because estradiol levels reflect follicular maturation, the measurement of estradiol as cited in the scientific literature has been used as a valuable tool in the assessment of sexual development in children, anovulation and/or amenorrhea, polycystic ovary syndrome and causes of infertility and menopause.
During in vitro fertilization, estradiol levels are routinely measured after gonadotropin stimulation to determine follicular status. Estradiol also affects areas other than reproductive tissues such as cardiovascular, immune and central nervous systems. For this reason estrogen has been investigated in the pathogenesis of cardiovascular disease, hormone-dependent cancers and osteoporotic fracture. Abnormally high levels in males are indicative of feminizing syndromes such as gynecomastia.
METHODOLOGY
The Access Sensitive Estradiol assay is a competitive binding immunoenzymatic assay. A sample is added to a reaction vessel with displacer and an anti-estradiol conjugate (anti-estradiol sheep monoclonal antibody conjugated to alkaline phosphatase in a MES-buffered protein solution). After incubation paramagnetic particles coated with estradiol analog are added. Estradiol in the sample competes with the estradiol analog on the paramagnetic particles for binding sites on a limited amount of specific anti-estradiol conjugate. The resulting estradiol analog-antibody complexes are bound on the solid phase.
After incubation in a reaction vessel, materials bound to the solid phase are held in a magnetic field while unbound materials are washed away. Then, the chemiluminescent substrate is added to the vessel and light generated by the reaction is measured with a luminometer. The light production is inversely proportional to the concentration of estradiol in the sample. The amount of analyte in the sample is determined from a stored, multi-point calibration curve.
TESTING PROCEDURE(S) PROCEDURE
1. Refer to the appropriate system manuals and/or Help system for a specific description of installation, start-up, principles of operation, system performance characteristics, operating instructions, calibration procedures, operational limitations and precautions, hazards, maintenance, and troubleshooting.
A. The system default unit of measure for sample results is pg/mL. To change sample reporting units to the International System of Units (SIunits), pmol/L, refer to the appropriate system manuals and/or Help system. To manually convert concentrations to the International System, multiply pg/mL by multiplication factor 3.671.
2. Mix contents of new (unpunctured) reagent packs by gently inverting pack several times before loading on the instrument. Do not invert open (punctured) packs.
3. Use thirty (30) µL of sample for each determination in addition to the sample container and system dead volumes when requesting the Access Sensitive Estradiol assay. Use one hundred and fifty-five (155) μL of sample in addition to the sample container and system dead volumes for each determination run with the DxI system onboard dilution feature (testname: dSNE2). Refer to the appropriate system manuals and/or Help system for the minimum sample volume required.
4. Refer to the appropriate system manuals and/or Help system for information on managing samples, configuring tests, requesting tests, and reviewing test results. A. Select SNSE2 as the test name for assaying samples containing estradiol concentrations up to the concentration of the Access Sensitive Estradiol S5 calibrator.
B. UniCel DxI users may use the UniCel DxI on board dilution feature (testname: dSNE2) for assaying samples containing estradiol concentrations greater than the Access Sensitive Estradiol S5 calibrator.
LIMITATIONS
1. For assays employing antibodies, the possibility exists for interference by heterophile antibodies in the patient sample. Patients who have been regularly exposed to animals or have received immunotherapy or diagnostic procedures utilizing immunoglobulins or immunoglobulin fragments may produce human anti-animal antibodies, e.g. HAMA, that interfere with immunoassays. Additionally, other antibodies such as human anti-goat antibodies may be present in patient samples. Such interfering antibodies may cause erroneous results. Carefully evaluate the results of patients suspected of having these antibodies.
2. Other potential interferences in the patient sample could be present and may cause erroneous results in immunoassays. Some examples that have been documented in literature include rheumatoid factor, endogenous alkaline phosphatase, fibrin, and proteins capable of binding to alkaline phosphatase.Carefully evaluate results if the sample is suspected of having these types of interferences.
3.TheSensitive Estradiol results should be interpreted in light of the total clinical presentation of the patient,including: symptoms, clinical history, data from additional tests, and other appropriate information.
RESULTS INTERPRETATION
Test results are determined automatically by the system software. The amount of analyte in the sample is determined from the measured light production by means of the stored calibration data. Test results can be reviewed using the appropriate screen. Refer to the appropriate system manuals and/or Help system for complete instructions on reviewing sample results.
REPORTING RESULTS
Samples can be accurately measured within the analytical range of the lower limit of detection and the highest calibrator value (approximately 15.0 - 5,200 pg/mL [55.1 - 19,089 pmol/L]).
• If a sample contains less than the lower limit of detection (LoD) for the assay, report the result as less than that value (i.e. <15.0pg/mL[55.1pmol/L]).When the DxI system on board dilution feature is used, the system will report results as less than 3,400 pg/mL (12,481 pmol/L).
• If a sample contains more than the stated value of the highest Access Sensitive Estradiol Calibrator (S5), report the result as greater than that value (i.e., > 5,200 pg/mL [> 19,089 pmol/L]). Alternatively, dilute one volume of sample with one volume of Access Sensitive Estradiol Calibrator S0.
Refer to the appropriate system manuals and/or Help system for instructions on entering a sample dilution in a test request. The system reports the results adjusted for the dilution.
Onboard Dilution Feature for use on UniCel DxI systems:
The DxI system onboard dilution feature automates the dilution process, using one volume of sample with one volume of Access Sample Diluent A, allowing samples to be quantitated up to approximately 10,400 pg/mL[38,178 pmol/L]. The system reports the results adjusted for the dilution.
EXPECTED RESULTS
1. Each laboratory should determine its own reference intervals appropriate to the laboratory’s patient population; including age of the patient.
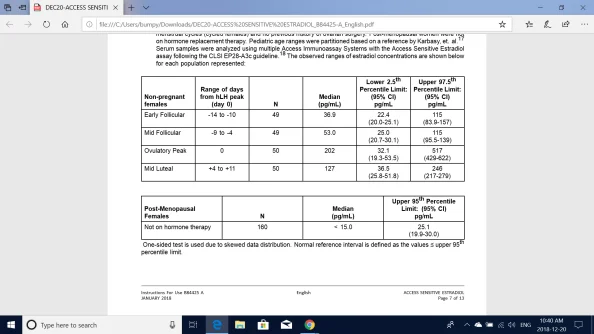
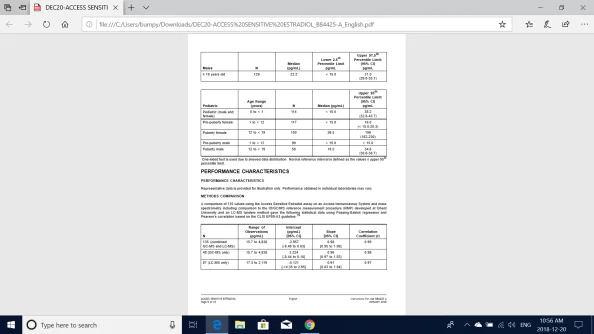
2. Estradiol concentrations were measured in human serum samples from apparently healthy adult male, female and pediatric subjects. Adult female subjects were not currently pregnant, not using hormonally based birth control in the three months prior to study, not using hormonal therapy in the six months prior to the study, had regular menstrual cycles (cycled females) and no previous history of ovarian surgery. Post-menopausal women were not on hormone replacement therapy. Pediatric age ranges were partitioned based on a reference by Karbasy,et. al.17 Serum samples were analyzed using multiple Access Immunoassay Systems with the Access Sensitive Estradiol assay following the CLSI EP28-A3c guideline. The observed ranges of estradiol concentrations are shown below for each population represented:





INTENDED USE
The Access Sensitive Estradiol assay is a paramagnetic particle, chemiluminescent immunoassay for the quantitative determination of estradiol levels in human serum and plasma using the Access Immunoassay Systems.
SUMMARY AND EXPLANATION
Estradiol (17β-estradiol) is a natural estrogen with a molecular mass of 272.4 daltons. Most circulating estradiol is strongly bound to sex hormone binding protein and loosely bound to albumin. It is estimated that only 1-5% of estradiol is free (unbound). In non-pregnant women,estradiol is secreted by the ovary and the corpus luteum. The adrenals and testes (in men) are also believed to secrete minute amounts of estradiol.
Estradiol levels are lowest at menses and into the early follicular phase and rise in the late follicular phase to a peak just prior to the hLH (human Luteinizing Hormone) surge, initiating ovulation. As the hLH peaks, the levels of estradiol decrease before rising again in the luteal phase. Endometrial growth is stimulated by estradiol and progesterone (secreted by the corpus luteum) in preparation for implantation of a fertilized egg. If conception does not occur, the secretion of estradiol and progesterone by the corpus luteum decreases, initiating menses.
Levels of estradiol are used to monitor ovulatory status. Because estradiol levels reflect follicular maturation, the measurement of estradiol as cited in the scientific literature has been used as a valuable tool in the assessment of sexual development in children, anovulation and/or amenorrhea, polycystic ovary syndrome and causes of infertility and menopause.
During in vitro fertilization, estradiol levels are routinely measured after gonadotropin stimulation to determine follicular status. Estradiol also affects areas other than reproductive tissues such as cardiovascular, immune and central nervous systems. For this reason estrogen has been investigated in the pathogenesis of cardiovascular disease, hormone-dependent cancers and osteoporotic fracture. Abnormally high levels in males are indicative of feminizing syndromes such as gynecomastia.
METHODOLOGY
The Access Sensitive Estradiol assay is a competitive binding immunoenzymatic assay. A sample is added to a reaction vessel with displacer and an anti-estradiol conjugate (anti-estradiol sheep monoclonal antibody conjugated to alkaline phosphatase in a MES-buffered protein solution). After incubation paramagnetic particles coated with estradiol analog are added. Estradiol in the sample competes with the estradiol analog on the paramagnetic particles for binding sites on a limited amount of specific anti-estradiol conjugate. The resulting estradiol analog-antibody complexes are bound on the solid phase.
After incubation in a reaction vessel, materials bound to the solid phase are held in a magnetic field while unbound materials are washed away. Then, the chemiluminescent substrate is added to the vessel and light generated by the reaction is measured with a luminometer. The light production is inversely proportional to the concentration of estradiol in the sample. The amount of analyte in the sample is determined from a stored, multi-point calibration curve.
TESTING PROCEDURE(S) PROCEDURE
1. Refer to the appropriate system manuals and/or Help system for a specific description of installation, start-up, principles of operation, system performance characteristics, operating instructions, calibration procedures, operational limitations and precautions, hazards, maintenance, and troubleshooting.
A. The system default unit of measure for sample results is pg/mL. To change sample reporting units to the International System of Units (SIunits), pmol/L, refer to the appropriate system manuals and/or Help system. To manually convert concentrations to the International System, multiply pg/mL by multiplication factor 3.671.
2. Mix contents of new (unpunctured) reagent packs by gently inverting pack several times before loading on the instrument. Do not invert open (punctured) packs.
3. Use thirty (30) µL of sample for each determination in addition to the sample container and system dead volumes when requesting the Access Sensitive Estradiol assay. Use one hundred and fifty-five (155) μL of sample in addition to the sample container and system dead volumes for each determination run with the DxI system onboard dilution feature (testname: dSNE2). Refer to the appropriate system manuals and/or Help system for the minimum sample volume required.
4. Refer to the appropriate system manuals and/or Help system for information on managing samples, configuring tests, requesting tests, and reviewing test results. A. Select SNSE2 as the test name for assaying samples containing estradiol concentrations up to the concentration of the Access Sensitive Estradiol S5 calibrator.
B. UniCel DxI users may use the UniCel DxI on board dilution feature (testname: dSNE2) for assaying samples containing estradiol concentrations greater than the Access Sensitive Estradiol S5 calibrator.
LIMITATIONS
1. For assays employing antibodies, the possibility exists for interference by heterophile antibodies in the patient sample. Patients who have been regularly exposed to animals or have received immunotherapy or diagnostic procedures utilizing immunoglobulins or immunoglobulin fragments may produce human anti-animal antibodies, e.g. HAMA, that interfere with immunoassays. Additionally, other antibodies such as human anti-goat antibodies may be present in patient samples. Such interfering antibodies may cause erroneous results. Carefully evaluate the results of patients suspected of having these antibodies.
2. Other potential interferences in the patient sample could be present and may cause erroneous results in immunoassays. Some examples that have been documented in literature include rheumatoid factor, endogenous alkaline phosphatase, fibrin, and proteins capable of binding to alkaline phosphatase.Carefully evaluate results if the sample is suspected of having these types of interferences.
3.TheSensitive Estradiol results should be interpreted in light of the total clinical presentation of the patient,including: symptoms, clinical history, data from additional tests, and other appropriate information.
RESULTS INTERPRETATION
Test results are determined automatically by the system software. The amount of analyte in the sample is determined from the measured light production by means of the stored calibration data. Test results can be reviewed using the appropriate screen. Refer to the appropriate system manuals and/or Help system for complete instructions on reviewing sample results.
REPORTING RESULTS
Samples can be accurately measured within the analytical range of the lower limit of detection and the highest calibrator value (approximately 15.0 - 5,200 pg/mL [55.1 - 19,089 pmol/L]).
• If a sample contains less than the lower limit of detection (LoD) for the assay, report the result as less than that value (i.e. <15.0pg/mL[55.1pmol/L]).When the DxI system on board dilution feature is used, the system will report results as less than 3,400 pg/mL (12,481 pmol/L).
• If a sample contains more than the stated value of the highest Access Sensitive Estradiol Calibrator (S5), report the result as greater than that value (i.e., > 5,200 pg/mL [> 19,089 pmol/L]). Alternatively, dilute one volume of sample with one volume of Access Sensitive Estradiol Calibrator S0.
Refer to the appropriate system manuals and/or Help system for instructions on entering a sample dilution in a test request. The system reports the results adjusted for the dilution.
Onboard Dilution Feature for use on UniCel DxI systems:
The DxI system onboard dilution feature automates the dilution process, using one volume of sample with one volume of Access Sample Diluent A, allowing samples to be quantitated up to approximately 10,400 pg/mL[38,178 pmol/L]. The system reports the results adjusted for the dilution.
EXPECTED RESULTS
1. Each laboratory should determine its own reference intervals appropriate to the laboratory’s patient population; including age of the patient.
2. Estradiol concentrations were measured in human serum samples from apparently healthy adult male, female and pediatric subjects. Adult female subjects were not currently pregnant, not using hormonally based birth control in the three months prior to study, not using hormonal therapy in the six months prior to the study, had regular menstrual cycles (cycled females) and no previous history of ovarian surgery. Post-menopausal women were not on hormone replacement therapy. Pediatric age ranges were partitioned based on a reference by Karbasy,et. al.17 Serum samples were analyzed using multiple Access Immunoassay Systems with the Access Sensitive Estradiol assay following the CLSI EP28-A3c guideline. The observed ranges of estradiol concentrations are shown below for each population represented:
Attachments
Last edited: