In my work collecting information for ExcelMale.com, I review abstracts daily on latest studies related to testosterone, men's health, nutrition, and more. I am always looking for studies that stand out and are no just repetitions of what we have seen before. Contrarian data to what I assume we know is what motivates me to read. In particular, I am looking for negative data and results in studies using testosterone. I have been using this hormone since 1993 to save my life and its quality and have not had any side effects. However, I know everyone is different and some people may have genetic or other variations that may make them susceptible to at least one side effect.
Good read:
What to do if you have a blood clot


The first time I read a paper than mentioned thrombosis risk in people on testosterone replacement (read abstract at the end of this article), my goal is to get in contact with the author. Dr Charles Glueck was kind to reply for my request for an interview to help me educate physicians and patients. He is a graduate from Harvard and Western Reserve Universities and has over 35 years of medical practice and have produced over 600 publications. He is currently the Medical Director of the Jewish Hospital Cholesterol Center. To say that he has credentials is an understatement.

I am impressed by his willingness to help anyone who may be concerned about this issue (he provides contact information below)
Here is the short interview:
Dr Glueck, Thank you so much for agreeing to educate my readers about your research.
Can you give give us a brief background of why you were interested in looking into thrombophilia and thrombosis in people on testosterone replacement therapy? Can you explain those terms to us?
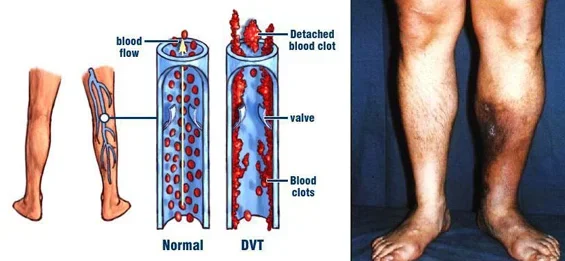
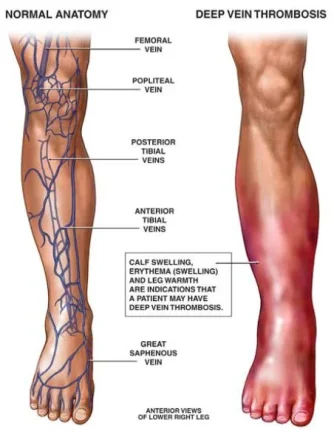
Dr Glueck: As physicians who deal with deep venous thrombosis (DVT) and pulmonary embolus (PE), as well as blood clots in the eyes (central retinal vein and central retinal artery thrombosis), and ischemic stroke, and arterial blood clots, we realized that many of our referrals had started exogenous conventional testosterone therapy before sustaining their blood clots. Because we were very experienced with the diagnosis of thrombophilia and hypofibrinolysis, we hypothesized that the exogenous testosterone was interacting with underlying coagulation disorders producing the blood clots. We have now proven this in multiple publications.
In your best estimate or opinion, what is the incidence of this problem in men on testosterone replacement?
Dr Glueck: The incidence of DVT-PE or other clots in men on T therapy is not known, but our best estimates are that about 1-2% of men taking T will develop blood clots related to underlying inherited clotting abnormalities or to acquired thrombophilia (the antiphospholipid antibody syndrome). These men who landed in the hospital with dangerous and potentially lethal blood clots in the deep veins of the legs or in the lungs developed these clots within three months of starting testosterone therapy. None of them knew previously that they had an inherited clotting disorder that put them at greater risk for developing clots, nor did their providers test them before putting them on testosterone therapy.
You suggest that "thrombophilia should be ruled out before administration of exogeneous testosterone". How can that be done and are the tests commercially available or research tools? You used these tests in your study: factor V Leiden heterozygosity, high factors VIII and XI, high homocysteine, low antithrombin III, the lupus anticoagulant, high anticardiolipin antibody lgG, and the hypofibrinolytic 4G4G mutation of the PAI-l gene. Should all be performed? Would these tests be reimbursed by insurance and, if not, what do you think the retail value would be?
Dr Glueck: The 4 tests we would do include Factor V Leiden, Prothrombin gene, Factor VIII and Factor XI, all routinely available commercially at Lab Corp and Quest (big national labs), and at almost all regional labs as well. In our experience these tests are routinely covered by insurance. If not covered, I would estimate that the cost would be expensive, $800.
You also suggest a link between high estradiol with thrombophilia. Can you explain this finding? Would anastrozole or other E2 inhibitor improve outcome if used with TRT?
Dr Glueck: We have data to show that when T is aromatized in the body to estradiol (E2), the high E2 may be the agent which directly interacts with the underlying thrombophilia to produce the clots. We do not have enough data to know whether Arimidex used to lower E2 would be protective, but we know that Arimidex alone is prothrombotic in all of the thrombophilias and hence, probably not a good idea.
In your opinion, should all men on TRT be on low dose aspirin?
Dr Glueck: Low dose aspirin would have no effect on the clotting events seen in men on T who have underlying thrombophilia and I would not recommend it.
Are you planning to do any further studies on this troubling issue?
Dr. Gluek: We are working hard to better understand this troubling issue. If any of your readers have had DVT-PE or other clots while taking exogenous T, or during hCG or clomid therapy to raise T, we would be glad to help them out with expert consultative advice free of charge. Have them contact us by email ([email protected]) or by phone (513-924-8250) fax (513-924-8273) and we will advise them on what blood samples to have drawn, and how to deal with their problem. All of their information will, of course, be entirely private and totally confidential. We will also be glad to work with their doctors in their local communities.
Thank you so much for your time and I will be contacting you in a few months to see if you have any updated data for us.
____________________________________________
ClincAppl Thromb Hemost. 2014 Jan;20(1):22-30. Epub 2013 Apr 23.
Testosterone, thrombophilia, and thrombosis.
Glueck CJ, Richardson-Royer C, Schultz R, Burger T, Labitue F, Riaz MK, Padda J, Bowe D, Goldenberg N, Wang P.
Abstract
We describe thrombosis, deep venous thrombosis (DVT) pulmonary embolism (PE; n = 9) and hip-knee osteonecrosis (n = 5) that developed after testosterone therapy (median 11 months) in 14 previously healthy patients (13 men and 1 woman; 13 Caucasian and 1 African American), with no antecedent thrombosis and previously undiagnosed thrombophilia-hypofibrinolysis. Of the 14 patients, 3 were found to be factor V Leiden heterozygotes, 3 had high factor VIII, 3 had plasminogen activator inhibitor 1 4G4G homozygosity, 2 had high factor XI, 2 had high homocysteine, 1 had low antithrombin III, 1 had the lupus anticoagulant, 1 had high anticardiolipin antibody Immunoglobulin G, and 1 had no clotting abnormalities. In 4 men with thrombophilia, DVT-PE recurred when testosterone was continued despite therapeutic international normalized ratio on warfarin. In 60 men on testosterone, 20 (33%) had high estradiol (E2 >42.6 pg/mL). When exogenous testosterone is aromatized to E2, and E2-induced thrombophilia is superimposed on thrombophilia-hypofibrinolysis, thrombosis occurs. The DVT-PE and osteonecrosis after starting testosterone are associated with previously undiagnosed thrombophilia-hypofibrinolysis. Thrombophilia should be ruled out before administration of exogenous testosterone.
Click here for part 2:
Article: Second Interview with Dr Charles Glueck About Testosterone and DVT
Good read:
What to do if you have a blood clot
The first time I read a paper than mentioned thrombosis risk in people on testosterone replacement (read abstract at the end of this article), my goal is to get in contact with the author. Dr Charles Glueck was kind to reply for my request for an interview to help me educate physicians and patients. He is a graduate from Harvard and Western Reserve Universities and has over 35 years of medical practice and have produced over 600 publications. He is currently the Medical Director of the Jewish Hospital Cholesterol Center. To say that he has credentials is an understatement.

I am impressed by his willingness to help anyone who may be concerned about this issue (he provides contact information below)
Here is the short interview:
Dr Glueck, Thank you so much for agreeing to educate my readers about your research.
Can you give give us a brief background of why you were interested in looking into thrombophilia and thrombosis in people on testosterone replacement therapy? Can you explain those terms to us?
Dr Glueck: As physicians who deal with deep venous thrombosis (DVT) and pulmonary embolus (PE), as well as blood clots in the eyes (central retinal vein and central retinal artery thrombosis), and ischemic stroke, and arterial blood clots, we realized that many of our referrals had started exogenous conventional testosterone therapy before sustaining their blood clots. Because we were very experienced with the diagnosis of thrombophilia and hypofibrinolysis, we hypothesized that the exogenous testosterone was interacting with underlying coagulation disorders producing the blood clots. We have now proven this in multiple publications.
In your best estimate or opinion, what is the incidence of this problem in men on testosterone replacement?
Dr Glueck: The incidence of DVT-PE or other clots in men on T therapy is not known, but our best estimates are that about 1-2% of men taking T will develop blood clots related to underlying inherited clotting abnormalities or to acquired thrombophilia (the antiphospholipid antibody syndrome). These men who landed in the hospital with dangerous and potentially lethal blood clots in the deep veins of the legs or in the lungs developed these clots within three months of starting testosterone therapy. None of them knew previously that they had an inherited clotting disorder that put them at greater risk for developing clots, nor did their providers test them before putting them on testosterone therapy.
You suggest that "thrombophilia should be ruled out before administration of exogeneous testosterone". How can that be done and are the tests commercially available or research tools? You used these tests in your study: factor V Leiden heterozygosity, high factors VIII and XI, high homocysteine, low antithrombin III, the lupus anticoagulant, high anticardiolipin antibody lgG, and the hypofibrinolytic 4G4G mutation of the PAI-l gene. Should all be performed? Would these tests be reimbursed by insurance and, if not, what do you think the retail value would be?
Dr Glueck: The 4 tests we would do include Factor V Leiden, Prothrombin gene, Factor VIII and Factor XI, all routinely available commercially at Lab Corp and Quest (big national labs), and at almost all regional labs as well. In our experience these tests are routinely covered by insurance. If not covered, I would estimate that the cost would be expensive, $800.
You also suggest a link between high estradiol with thrombophilia. Can you explain this finding? Would anastrozole or other E2 inhibitor improve outcome if used with TRT?
Dr Glueck: We have data to show that when T is aromatized in the body to estradiol (E2), the high E2 may be the agent which directly interacts with the underlying thrombophilia to produce the clots. We do not have enough data to know whether Arimidex used to lower E2 would be protective, but we know that Arimidex alone is prothrombotic in all of the thrombophilias and hence, probably not a good idea.
In your opinion, should all men on TRT be on low dose aspirin?
Dr Glueck: Low dose aspirin would have no effect on the clotting events seen in men on T who have underlying thrombophilia and I would not recommend it.
Are you planning to do any further studies on this troubling issue?
Dr. Gluek: We are working hard to better understand this troubling issue. If any of your readers have had DVT-PE or other clots while taking exogenous T, or during hCG or clomid therapy to raise T, we would be glad to help them out with expert consultative advice free of charge. Have them contact us by email ([email protected]) or by phone (513-924-8250) fax (513-924-8273) and we will advise them on what blood samples to have drawn, and how to deal with their problem. All of their information will, of course, be entirely private and totally confidential. We will also be glad to work with their doctors in their local communities.
Thank you so much for your time and I will be contacting you in a few months to see if you have any updated data for us.
____________________________________________
ClincAppl Thromb Hemost. 2014 Jan;20(1):22-30. Epub 2013 Apr 23.
Testosterone, thrombophilia, and thrombosis.
Glueck CJ, Richardson-Royer C, Schultz R, Burger T, Labitue F, Riaz MK, Padda J, Bowe D, Goldenberg N, Wang P.
Abstract
We describe thrombosis, deep venous thrombosis (DVT) pulmonary embolism (PE; n = 9) and hip-knee osteonecrosis (n = 5) that developed after testosterone therapy (median 11 months) in 14 previously healthy patients (13 men and 1 woman; 13 Caucasian and 1 African American), with no antecedent thrombosis and previously undiagnosed thrombophilia-hypofibrinolysis. Of the 14 patients, 3 were found to be factor V Leiden heterozygotes, 3 had high factor VIII, 3 had plasminogen activator inhibitor 1 4G4G homozygosity, 2 had high factor XI, 2 had high homocysteine, 1 had low antithrombin III, 1 had the lupus anticoagulant, 1 had high anticardiolipin antibody Immunoglobulin G, and 1 had no clotting abnormalities. In 4 men with thrombophilia, DVT-PE recurred when testosterone was continued despite therapeutic international normalized ratio on warfarin. In 60 men on testosterone, 20 (33%) had high estradiol (E2 >42.6 pg/mL). When exogenous testosterone is aromatized to E2, and E2-induced thrombophilia is superimposed on thrombophilia-hypofibrinolysis, thrombosis occurs. The DVT-PE and osteonecrosis after starting testosterone are associated with previously undiagnosed thrombophilia-hypofibrinolysis. Thrombophilia should be ruled out before administration of exogenous testosterone.
Click here for part 2:
Article: Second Interview with Dr Charles Glueck About Testosterone and DVT
Last edited:

















