Nelson Vergel
Founder, ExcelMale.com
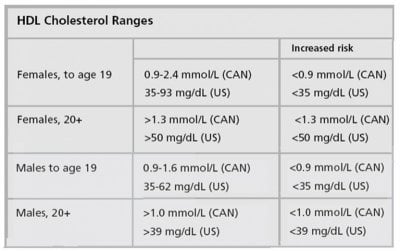
This HDL lowering effect is seen at higher testosterone doses.
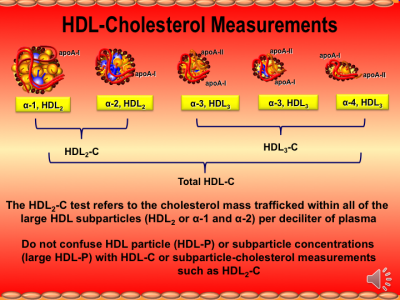
Relationship of plasma HDL-Cholesterol to testosterone, estradiol, and sex-hormone-binding globulin levels in men and women
James Semmens et al.
Metabolism
Volume 32, Issue 5, May 1983, Pages 428–432
Abstract
The significance of sex hormone levels in determining variation in high-density lipoprotein cholesterol (HDL-C) concentrations was studied in healthy Seventh Day Adventists (vegetarians) and Mormons. These groups were selected to avoid the confounding effects of alcohol consumption and cigarette smoking on HDL-C concentrations. Multivariate analysis showed that testosterone has a strong negative association with HDL-C in men (t = 3.99, P < 0.001) and women (t = 2.04, P < 0.05) when controlled for other variables including the concentration of sex-hormone-binding globulin (SHBG). Sex-hormone-binding globulin showed an independent positive association with HDL-C in men (P < 0.001). We postulate that the sex hormones affect HDL-C levels by regulating the activities of two important enzymes involved in the production and catabolism of HDL, namely, lipoprotein lipase and hepatic endothelial lipase. Other factors contributing independently to variation in HDL-C levels in this study were, in men, age and triglyceride, and in women, apoprotein-HDL, triglyceride, systolic blood pressure, heart rate, body mass index, and triceps skinfold thickness. Plasma estradiol concentrations were not significantly associated in either sex.
Testosterone (or Anastrozole?) Decreased my HDL: What Can I Do?
How to Increase Good Cholesterol (HDL)
Relationship of plasma HDL-Cholesterol to testosterone, estradiol, and sex-hormone-binding globulin levels in men and women

James Semmens et al.
Metabolism
Volume 32, Issue 5, May 1983, Pages 428–432
Abstract
The significance of sex hormone levels in determining variation in high-density lipoprotein cholesterol (HDL-C) concentrations was studied in healthy Seventh Day Adventists (vegetarians) and Mormons. These groups were selected to avoid the confounding effects of alcohol consumption and cigarette smoking on HDL-C concentrations. Multivariate analysis showed that testosterone has a strong negative association with HDL-C in men (t = 3.99, P < 0.001) and women (t = 2.04, P < 0.05) when controlled for other variables including the concentration of sex-hormone-binding globulin (SHBG). Sex-hormone-binding globulin showed an independent positive association with HDL-C in men (P < 0.001). We postulate that the sex hormones affect HDL-C levels by regulating the activities of two important enzymes involved in the production and catabolism of HDL, namely, lipoprotein lipase and hepatic endothelial lipase. Other factors contributing independently to variation in HDL-C levels in this study were, in men, age and triglyceride, and in women, apoprotein-HDL, triglyceride, systolic blood pressure, heart rate, body mass index, and triceps skinfold thickness. Plasma estradiol concentrations were not significantly associated in either sex.
Testosterone (or Anastrozole?) Decreased my HDL: What Can I Do?
How to Increase Good Cholesterol (HDL)
Last edited: