madman
Super Moderator
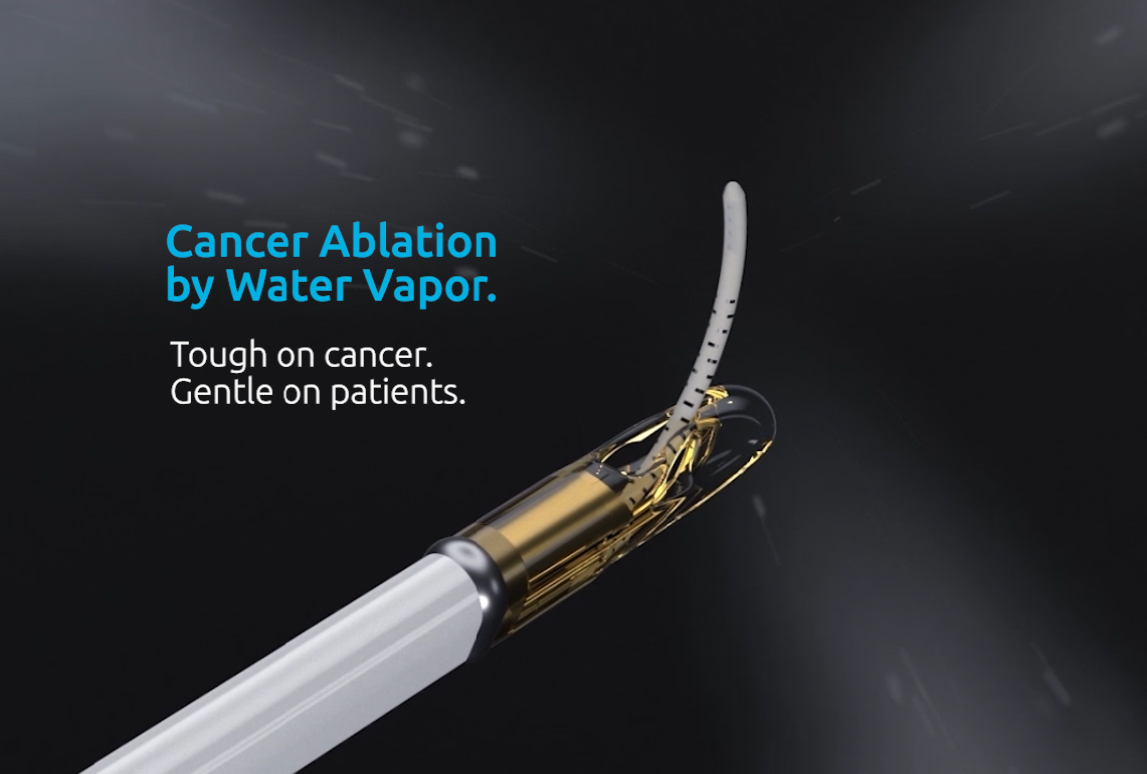
Study launches exploring water vapor ablation in localized prostate cancer
“Water vapor therapy shows great promise to provide patients with another option to proactively manage their cancer risk while preserving quality of life,” said Naveen Kella, MD.
The VAPOR 2 trial evaluates the Vanquish Water Vapor Ablation Device for intermediate-risk, localized prostate cancer. Dr. Naveen Kella administered the treatment in San Antonio. Water vapor therapy shows promise in managing cancer risk and preserving patients' quality of life by reducing side effects from traditional treatments.
VAPOR 2 trial
*VAPOR 2 study has 235 enrolled patients receiving water vapor ablation.
*Patients must be at least 50 years old with a life expectancy of ≥10 years to enroll.
*Prostate size at baseline should be 20 ccs to 80 cc, PSA level ≤15 ng/ml, and cancer stage ≤T2c.
*Exclusion criteria include certain prostate conditions, prior surgeries, and hormonal medications.
*Primary efficacy endpoint: freedom from systemic disease, therapy, salvage therapy, and GGG≥2.
*Key secondary endpoint: rate of patients without impotence.
*Estimated primary completion date: April 2027
Water vapor is a powerful and gentle tool, a step closer to becoming a groundbreaking treatment for urologists and patients. Michael Hoey, the founder, and CTO of Francis Medical, expressed excitement about the milestone, thanking employees, investors, patients, and physicians for their support. Michael Kujak, the president, and CEO of Francis Medical, shared confidence that this technology will be the preferred first-line treatment for men and their doctors.