madman
Super Moderator
Effect of Testosterone Replacement Therapy on Sexual Function and Hypogonadal Symptoms in Men with Hypogonadism
AbstractContext. Few long-term randomized trials have evaluated the efficacy of testosterone replacement therapy (TRT) in improving sexual function and hyp
Within the larger TRAVERSE trial, the Sexual Function Study focuses on the impact of TRT on sexual activity, hypogonadal symptoms, libido, and erectile function in men with low libido.
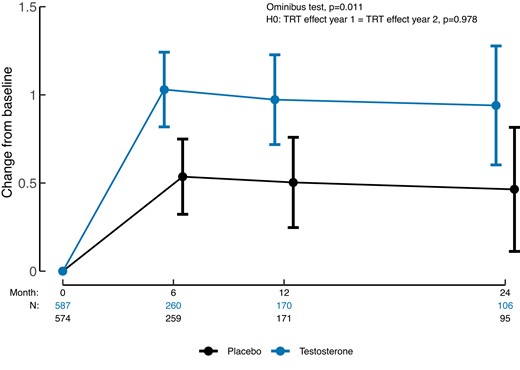
The study involves 5204 men aged 45 to 80 with low testosterone, hypogonadal symptoms, and cardiovascular risk. Among these, 1161 men with low libido were included in the Sexual Function Study, with half receiving 1.62% testosterone gel and the others a placebo gel. The primary outcome measured is the change in sexual activity score, and secondary outcomes include hypogonadal symptoms, erectile function, and sexual desire.
Results show that TRT leads to a significantly greater improvement in sexual activity compared to placebo at 6 and 12 months, with the effect maintained at 24 months. TRT also improves hypogonadal symptoms and sexual desire, but there is no substantial effect on erectile function.
In conclusion, the study demonstrates that TRT over two years improves sexual activity, hypogonadal symptoms, and sexual desire in middle-aged and older men with hypogonadism and low libido, while not significantly affecting erectile function.











