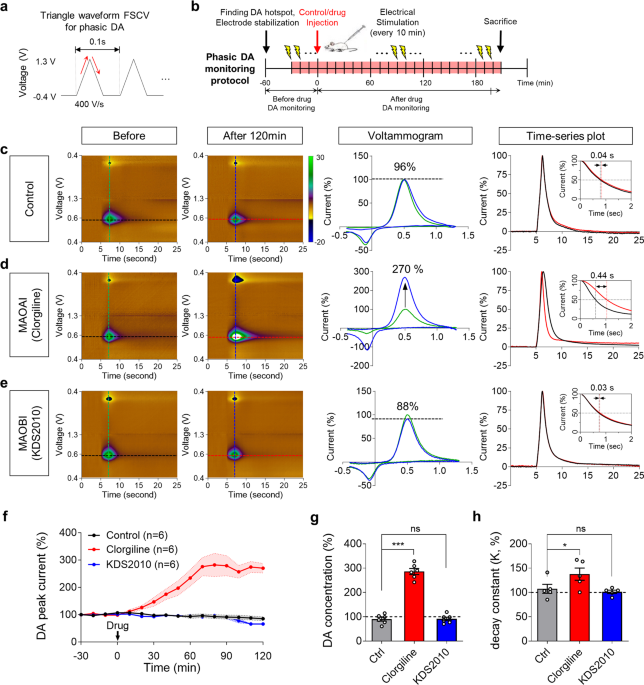
BadassBlues
Well-Known Member

The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015) - PubMed
Deprenyl/Selegiline (DEP), created by Joseph Knoll in the 1960s, registered in more than 60 countries to treat Parkinson's disease, Alzheimer's disease, major depressive disorder; and used as an anti-aging drug, achieved its place in research and therapy as the first selective inhibitor of...
The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015)
I Miklya 1Affiliations expand
- PMID: 27480491
- DOI: 10.1038/mp.2016.127






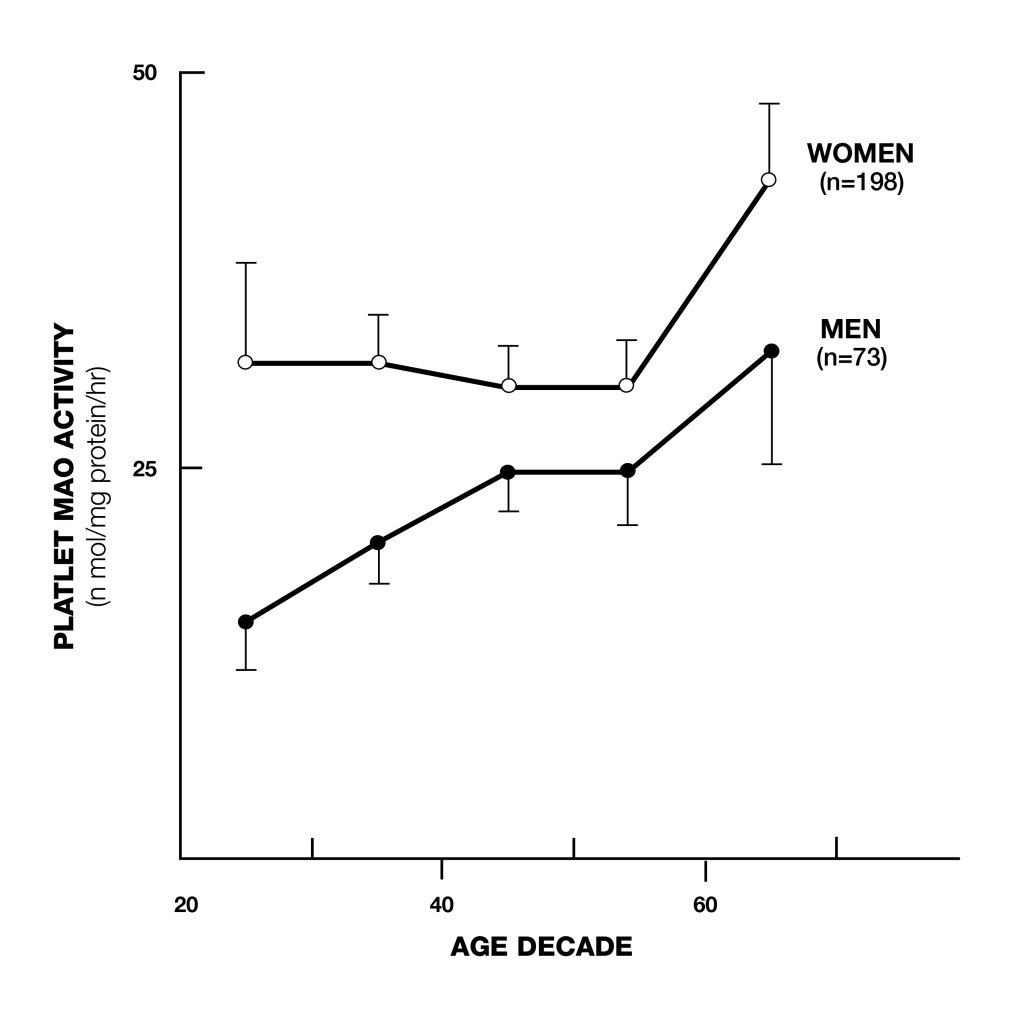 Figure 1: Platelet MAO-B increases with age
Figure 1: Platelet MAO-B increases with age
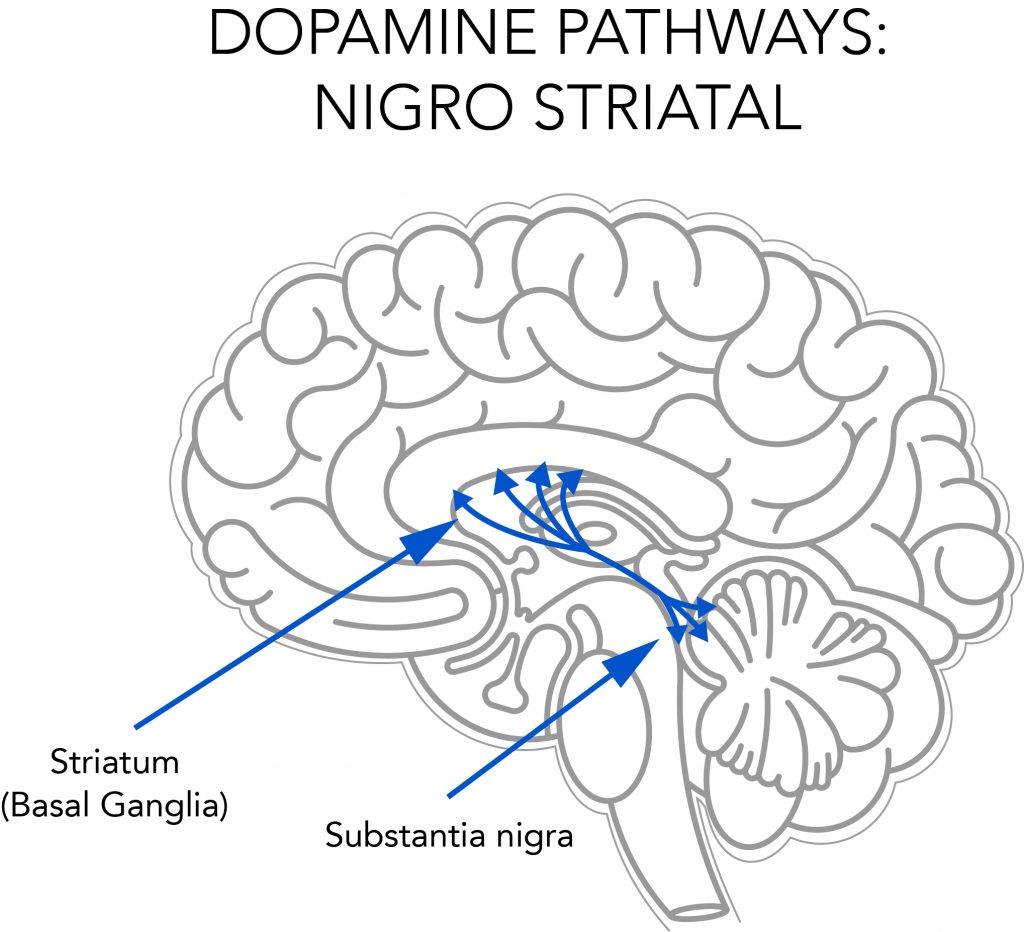 Figure 2: Straital dopaminergic neurons. The striatum is the main input nucleus of the basal ganglia and a key neural substrate for procedural learning and memory.
Figure 2: Straital dopaminergic neurons. The striatum is the main input nucleus of the basal ganglia and a key neural substrate for procedural learning and memory.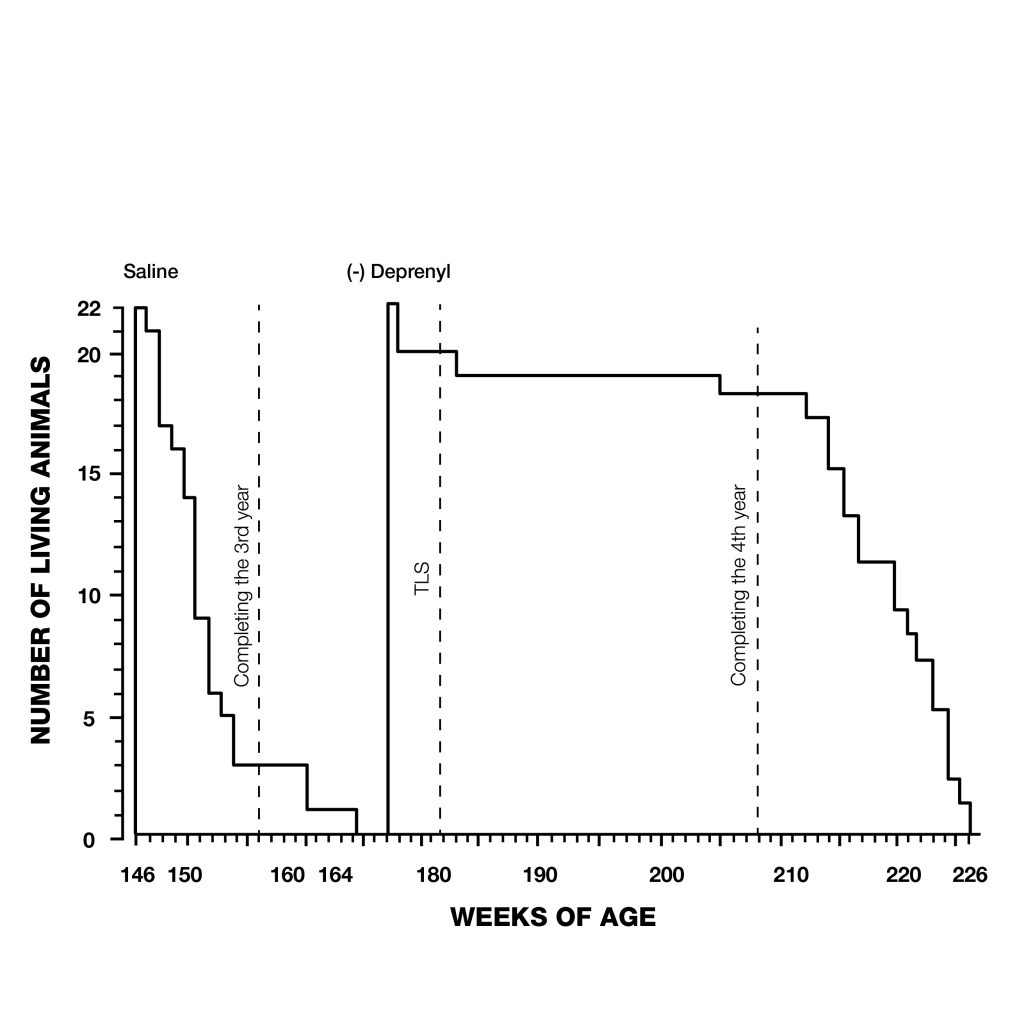 Figure 3: Extension of maximum lifespan in rats injected with deprenyl 3 times/week, beginning at the age of 24 months. Note that all the control rats died before the first deprenyl-treated rat died.
Figure 3: Extension of maximum lifespan in rats injected with deprenyl 3 times/week, beginning at the age of 24 months. Note that all the control rats died before the first deprenyl-treated rat died.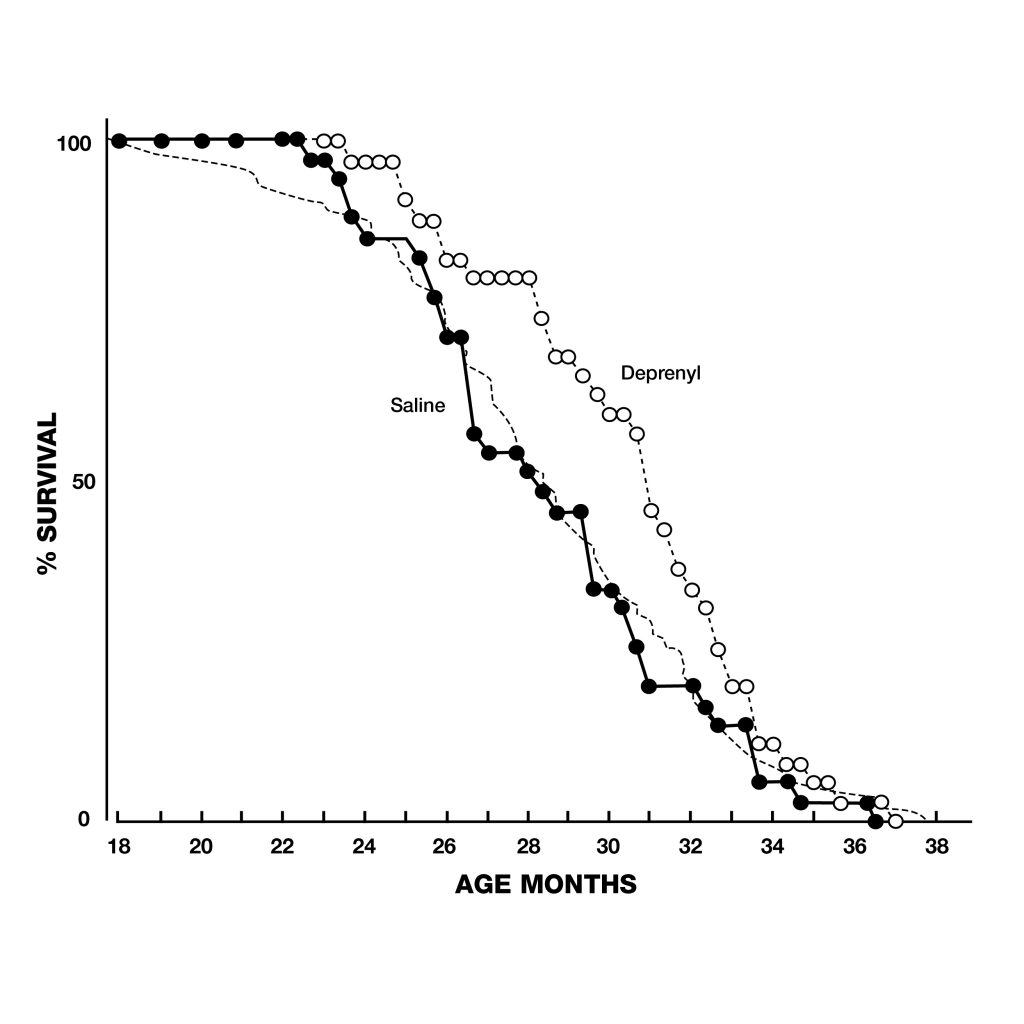 Figure 4: Extension of lifespan of deprenyl-treated rats (open circles) from the Tokyo Metropolitan institute of Gerontology. Treatment began at 18 months of age, resulted in 34% increase in the remaining life expectancy after 24 months.
Figure 4: Extension of lifespan of deprenyl-treated rats (open circles) from the Tokyo Metropolitan institute of Gerontology. Treatment began at 18 months of age, resulted in 34% increase in the remaining life expectancy after 24 months.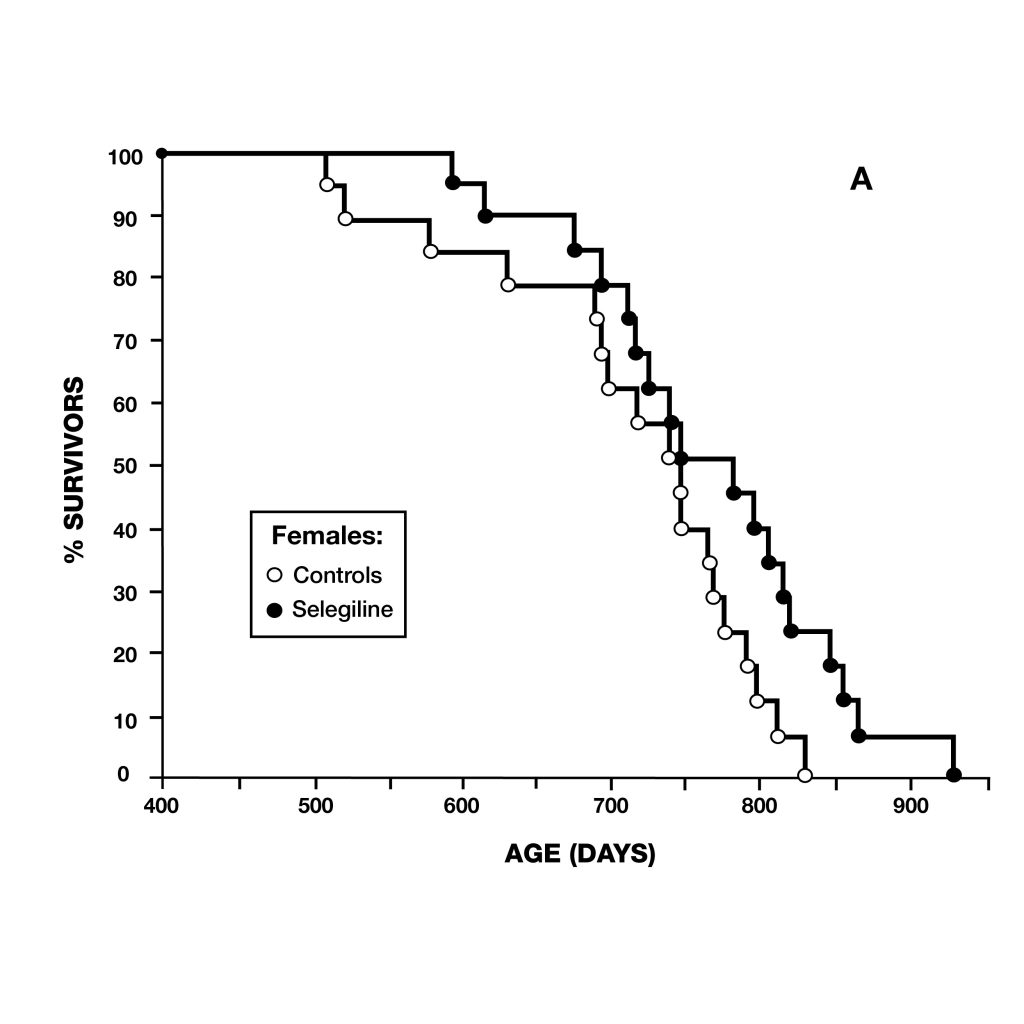
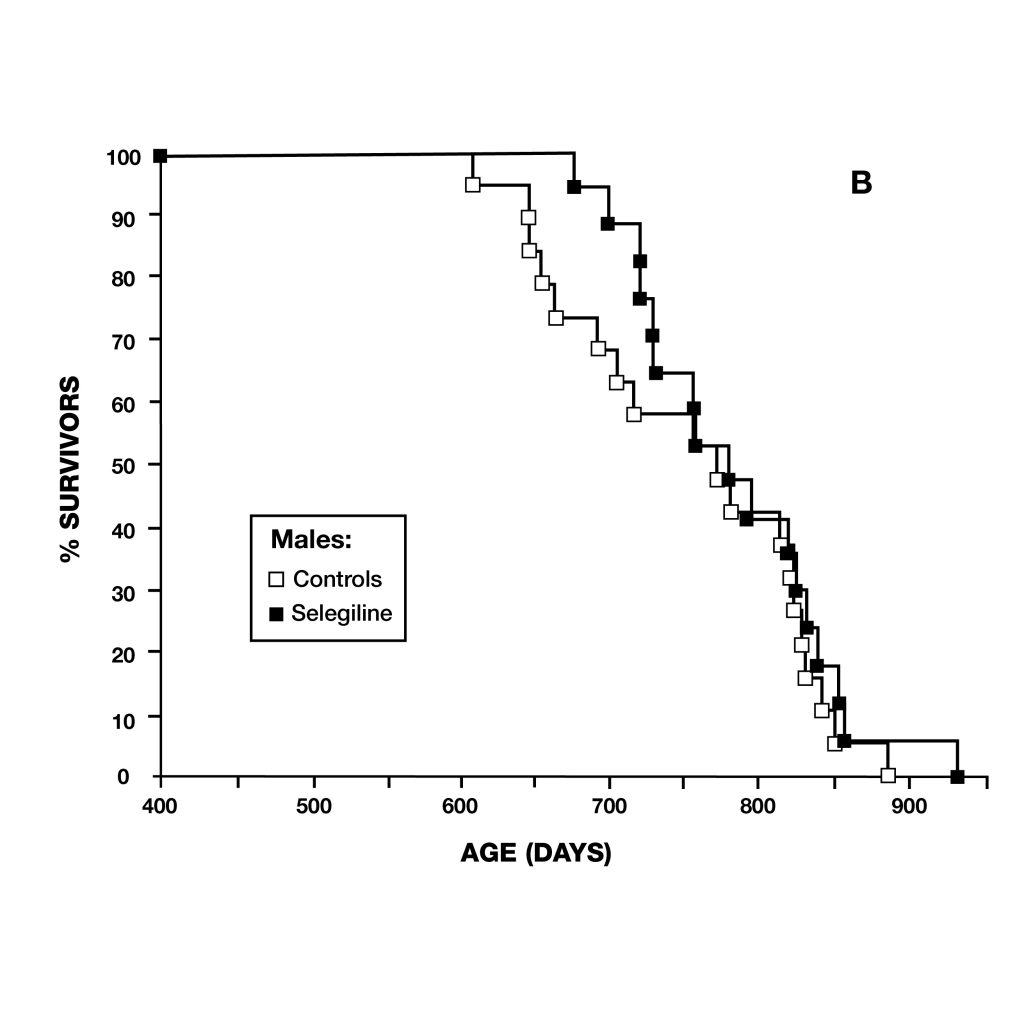 Figure 5: Survival curves of female (A) and male (B) syrian hamsters. Female hamsters normally have shorter lifespans than males. After deprenyl treatment, the difference of lifespan between male and female hamster disappeared.
Figure 5: Survival curves of female (A) and male (B) syrian hamsters. Female hamsters normally have shorter lifespans than males. After deprenyl treatment, the difference of lifespan between male and female hamster disappeared.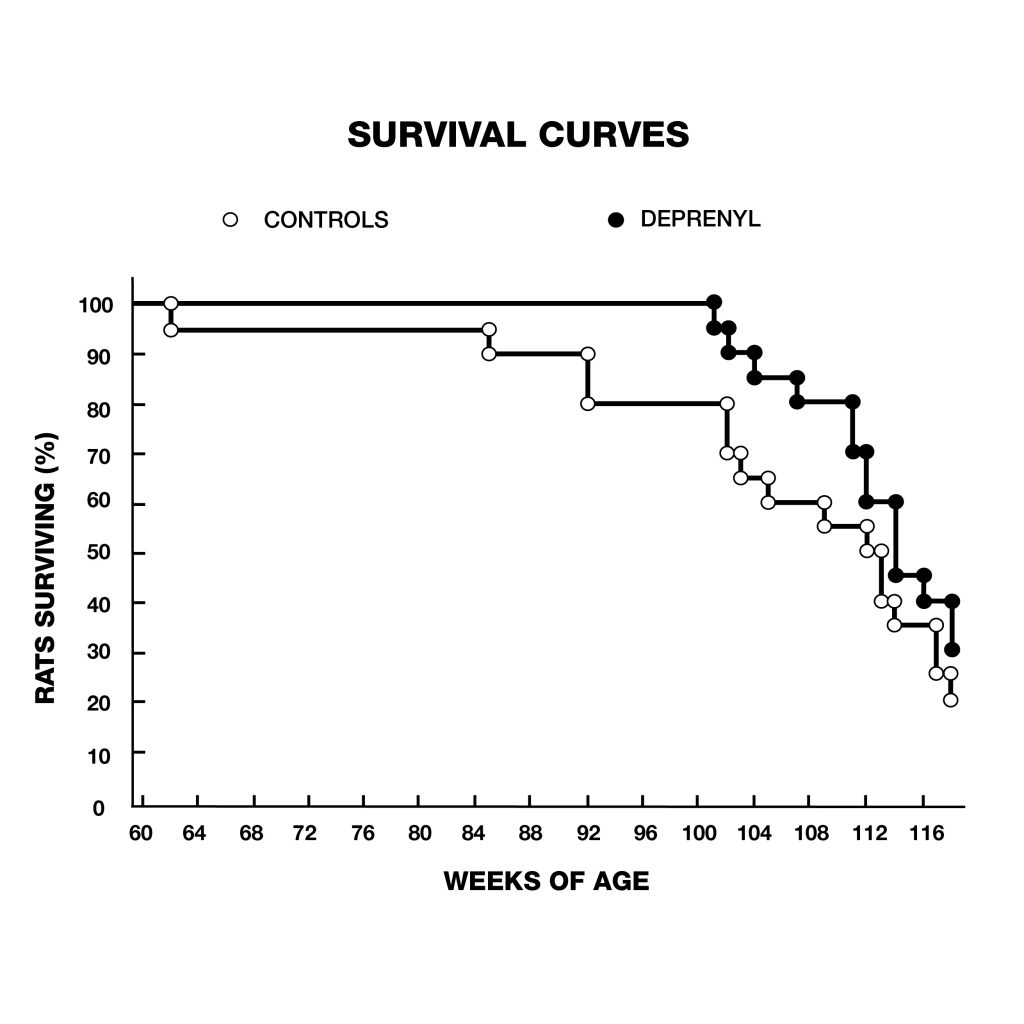 Figure 6: extension of lifespan of male rats treated with deprenyl in their drinking water, compared to controls. Treatment was begun at 54 weeks of age. Deprenyl-treated rats exhibited higher survival rates at all time points after 62 weeks of age.
Figure 6: extension of lifespan of male rats treated with deprenyl in their drinking water, compared to controls. Treatment was begun at 54 weeks of age. Deprenyl-treated rats exhibited higher survival rates at all time points after 62 weeks of age.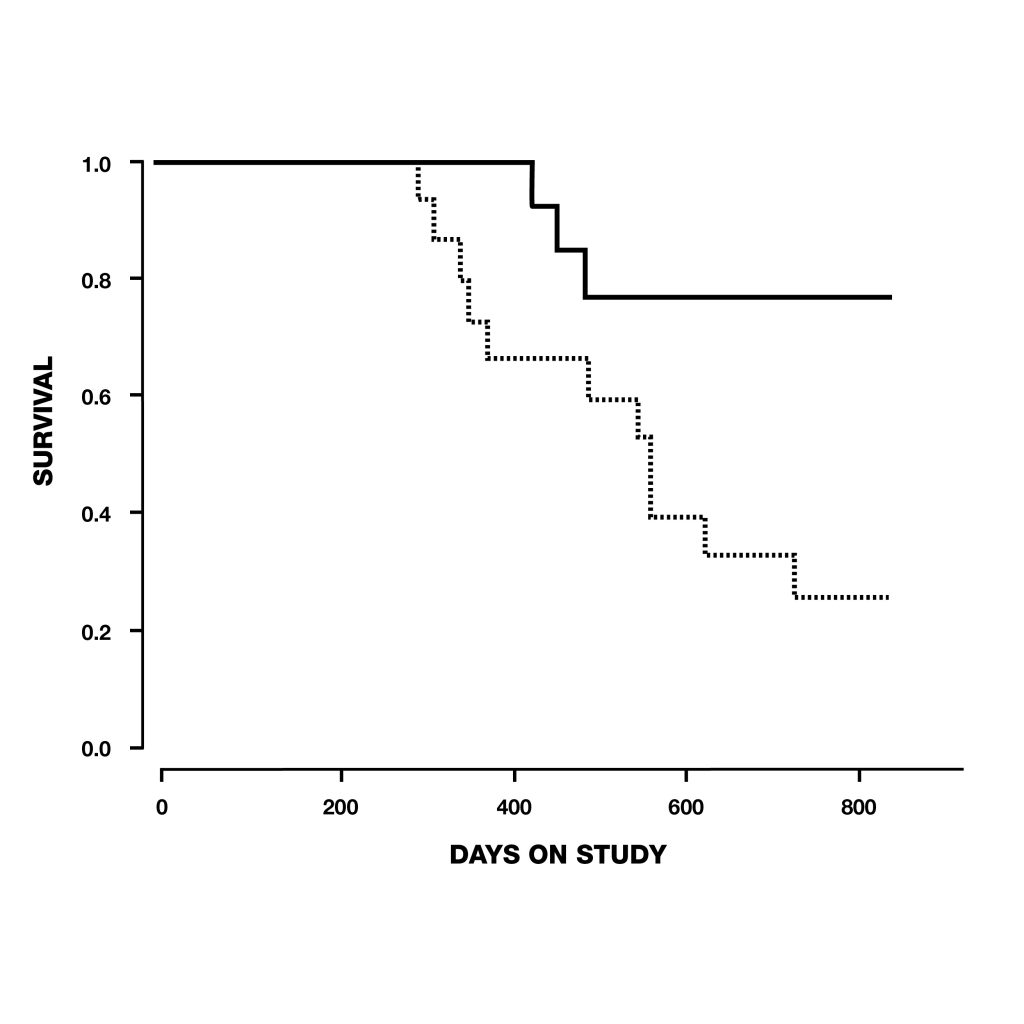 Figure 7: Survival of dogs, between 10 and 15 years old at the start of the study, treated with deprenyl for at least six months. Note that by the time the first deprenyl treated dog died on day 427, five of the placebo-treated dogs has already died.
Figure 7: Survival of dogs, between 10 and 15 years old at the start of the study, treated with deprenyl for at least six months. Note that by the time the first deprenyl treated dog died on day 427, five of the placebo-treated dogs has already died.