(Endoluten), and vascular health (Ventfort) all contribute to skin quality from the inside out.
The research behind Khavinson peptides
Skepticism is reasonable when encountering claims about life extension and disease prevention. What sets Khavinson peptides apart from many longevity compounds is the sheer volume and duration of supporting research. Over 775 published papers spanning four decades, animal studies across multiple species, cell culture experiments with measurable molecular endpoints, and multi-year human clinical trials involving hundreds of participants. Researchers accustomed to evaluating
peptide research will recognize that few compounds in the entire peptide field have this depth of longitudinal data. Brain bioregulators like
Cortagen, immune regulators like
Thymalin, and pineal activators like
Epitalon each have dedicated research archives spanning decades. This section examines the key evidence areas that underpin the bioregulator system.
Telomere lengthening and telomerase activation
Telomere research is where Khavinson peptides have generated the most international attention. Telomeres are the protective caps at the ends of chromosomes. They shorten with each cell division, and when they become critically short, cells enter senescence and stop dividing. This process is one of the fundamental mechanisms of biological aging. Telomerase is the enzyme that rebuilds telomeres, but its expression is largely silenced in adult somatic cells.
Epitalon reactivates it.
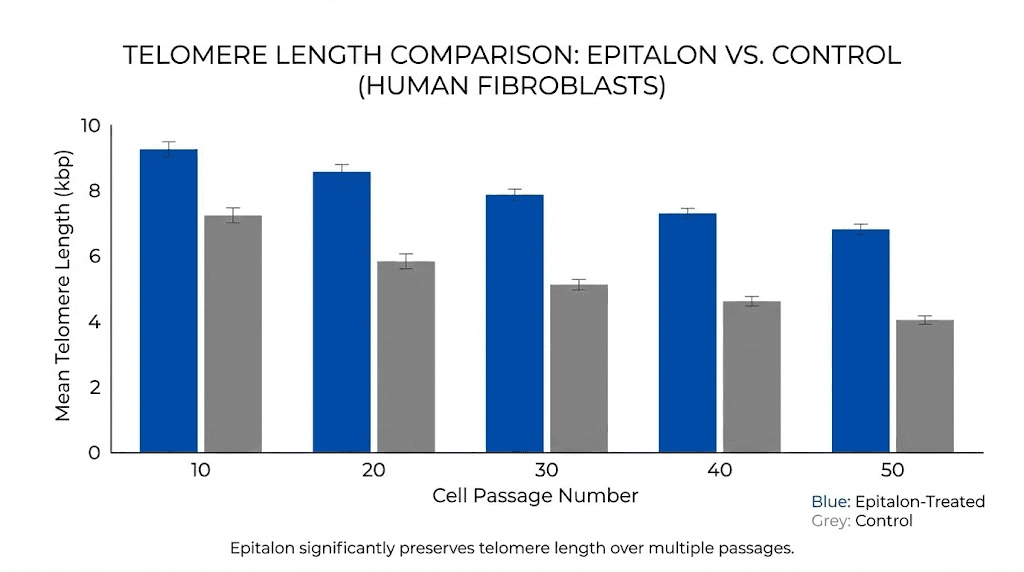
In the foundational study published in 2003, adding the Epithalon peptide to telomerase-negative human fetal fibroblast cultures induced expression of the catalytic subunit of telomerase, increased enzymatic activity, and produced measurable telomere elongation. The cells were able to continue dividing past the Hayflick limit, the natural replication boundary that normally restricts cell division. Control cells stopped dividing at the 34th passage. Epitalon-treated cells continued past the 44th passage with a 2.4-fold increase in telomere length. These are not marginal effects.
The recent study published in
Biogerontology provided even more detailed molecular data. Researchers treated breast cancer cell lines 21NT and BT474, as well as normal epithelial and fibroblast cells, with Epitalon. qPCR and immunofluorescence analysis demonstrated dose-dependent telomere length extension in normal cells through hTERT and telomerase upregulation.
The hTERT mRNA expression increased 12-fold in normal cells. Critically, the study also revealed that in cancer cells, telomere extension occurred through a completely different pathway called ALT (Alternative Lengthening of Telomeres), not through telomerase activation. This finding has significant implications for safety, suggesting Epitalon does not stimulate telomerase in cells that are already cancerous. In human clinical studies, both Epitalon and Epithalamin significantly increased telomere lengths in blood cells of patients aged 60-65 and 75-80. Some studies reported telomere length increases averaging 33.3%, though individual results varied based on baseline telomerase activity, cellular age, and metabolic factors. For detailed protocols, the
Epitalon dosage guide covers clinical dosing ranges and cycle recommendations.
Lifespan extension studies
Animal lifespan studies provide some of the most compelling evidence for Khavinson peptides as genuine geroprotectors. Long-term administration of bioregulator peptides to mice, rats, and
Drosophila melanogaster (fruit flies) consistently produced 20-40% increases in average lifespan. These are not small numbers in longevity research. To put this in perspective, caloric restriction, one of the most well-established life extension interventions in animal models, typically produces lifespan increases of 20-30% in rodents.
The data showed that peptide treatment slowed age-related changes in biomarkers of aging and suppressed development of both spontaneous and chemically induced tumors. Geroprotective effects of Thymalin and Epithalamin were dynamically tested over 14-20 year observation periods in animal models and proved highly effective. The indices of the two major homeostatic systems, neuroendocrine and immune, were restored to their normal values in middle-aged and elderly subjects. This restoration of homeostatic function is the key mechanism behind the lifespan extension. The animals did not just live longer. They lived healthier. Their immune function improved. Their hormonal regulation normalized. Their tumor incidence decreased. This pattern of healthspan extension alongside lifespan extension is exactly what
longevity researchers hope to achieve.
Additional animal studies expanded on these findings in specific organ systems. Epitalon treatment in aging mice significantly reduced the incidence of chromosomal aberrations in both wild-type mice and mice with accelerated aging phenotypes. This chromosomal protection is consistent with telomere lengthening effects. In aging rats, Epitalon increased the activities of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, and glutathione-S-transferase, demonstrating that the peptide enhances cellular defense mechanisms against oxidative damage. Perhaps most remarkably, Epitalon reduced the number of spontaneous tumors and metastases in a study of one-year-old female mice, reinforcing the anti-carcinogenic properties observed across multiple experimental models. The breadth of benefits across different organ systems and disease endpoints supports the fundamental thesis that restoring gene expression through bioregulators creates cascading improvements throughout the body. Compounds like
SS-31, which target mitochondrial function, and
MOTS-c, which modulates metabolic pathways, address aging through different mechanisms. But Khavinson peptides address aging at its source: the gradual silencing of the genes that keep tissues functioning properly.
The 266-person clinical trial
The most important piece of human evidence for Khavinson peptides comes from a clinical study published in
Neuroendocrinology Letters in 2003. Researchers at the Saint Petersburg Institute of Bioregulation and Gerontology and the Institute of Gerontology of the Ukrainian Academy of Medical Sciences clinically assessed the geroprotective effects of Thymalin and Epithalamin in 266 elderly patients over a period of six to eight years.
The results were extraordinary.
Bioregulator treatment normalized basic functions of the human organism, improving indices of cardiovascular, endocrine, immune, and nervous systems, as well as homeostasis and metabolism. Disease incidence dropped dramatically. Acute respiratory disease incidence decreased 2.0-2.4 fold. Incidence of ischemic heart disease, hypertension, deforming osteoarthrosis, and osteoporosis all declined compared to controls. But the mortality data is what captured global attention. The mortality rate decreased 2.0-2.1 fold in the Thymalin-treated group. It decreased 1.6-1.8 fold in the Epithalamin-treated group. Combined treatment with both peptides produced a 2.5-fold mortality reduction. And the most remarkable finding of all: patients who received both Thymalin and Epithalamin annually for six consecutive years showed a 4.1-fold reduction in mortality compared to controls. All agents were injected intramuscularly at 10mg daily for 10 days (100mg per course), and the trial was described as double-blind. These bioregulators were applied in combination with standard therapy for corresponding clinical indications.
Context is important here. These results have not been independently replicated by research groups outside Khavinson organization. Every preclinical and clinical study discussed was conducted by his group in Russia. More international validation is needed, and the broader scientific community has called for larger-scale, independent clinical trials. However, the consistency of the data across multiple endpoints, the long observation period, and the large sample size make this trial noteworthy. For researchers evaluating
peptide safety and risks, the clinical safety data from this trial is also relevant, showing no significant adverse effects from long-term bioregulator use.
Anti-cancer properties
Multiple studies from Khavinson research group demonstrated that bioregulator peptides possess anti-carcinogenic properties. Long-term treatment with peptide preparations suppressed development of both spontaneous tumors and tumors induced by chemical or radiation carcinogens in rodent models. Epithalamin specifically increases melatonin production by the pineal gland, which itself has well-documented anti-cancer properties. Elevated melatonin inhibits tumor cell proliferation, enhances immune surveillance, and reduces oxidative DNA damage.
At the molecular level, the anti-aging effects of Khavinson peptides overlap significantly with anti-cancer mechanisms. The AEDG peptide (Epitalon) and KED peptide both decreased expression of p16 and p21, two senescence markers that are also involved in tumor suppressor pathways. AEDG decreased p16 and p21 mRNA expression by 1.56-2.44 times compared to controls. KED decreased them by 1.82-3.23 times. Cartalax reduced p53 expression by 25% while upregulating SIRT6 pathways. These effects suggest that bioregulator peptides maintain cellular youth without promoting uncontrolled growth. The recent
Biogerontology study on Epitalon reinforced this, showing that the peptide activates different pathways in normal versus cancer cells, using telomerase upregulation in normal cells but ALT activation in cancer cell lines. This selectivity is remarkable and addresses one of the primary safety concerns about telomerase-activating compounds. For deeper exploration of the relationship between aging and cellular health, the
peptide research database at
SeekPeptides provides additional study summaries.
How to use Khavinson peptides
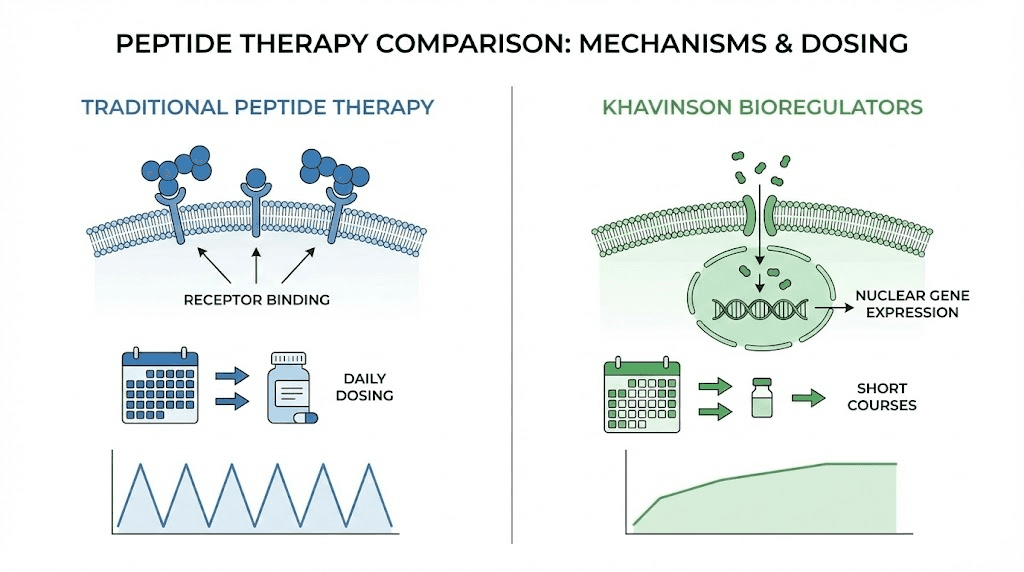
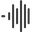
Understanding the science is one thing. Implementing an effective protocol is another. Khavinson peptide bioregulators use dosing strategies that differ substantially from most other
peptide dosing approaches. The protocols are built around short, intensive courses rather than continuous daily administration. This section covers every major protocol variation, from basic maintenance to comprehensive multi-organ stacking.
Maintenance protocol
The maintenance protocol is designed for healthy individuals who want to preserve organ function and prevent age-related decline. It is the simplest entry point into bioregulator therapy and is recommended as a starting point for anyone over 35. Those already following
beginner peptide protocols will find bioregulator dosing refreshingly straightforward compared to the precise measurements required for
injectable peptides.
The standard maintenance approach involves taking 2 capsules daily for 10 days. That is 20 capsules total, equivalent to one pack of most commercial bioregulator products. This course is then repeated six months later. For general preventive purposes, two to three 10-day courses per year are sufficient to maintain systemic health. The key insight is that bioregulators have a prolonged aftereffect. Unlike most supplements that only work while you are taking them, the gene expression changes triggered by bioregulators persist for months after the course ends. Cytomaxes produce effects lasting 6-12 months. This means short, intense courses followed by long breaks is not a limitation. It is the designed approach. For those tracking their
peptide cycles, bioregulators fit a distinctly different pattern than most research peptides.
Therapeutic protocol
The therapeutic protocol is designed for individuals with existing health conditions, those over 45, or anyone requiring more intensive bioregulatory support. The dosing increases significantly compared to maintenance.
In the therapeutic approach, the standard course involves 2 capsules daily for 30 days. This extended duration allows deeper tissue saturation and more sustained gene expression modulation. After the 30-day course, a break period follows before repeating. The frequency of therapeutic courses increases with age and severity of conditions. Researchers working with
peptide dosage charts should note that bioregulator dosing is substantially different from typical injectable peptide protocols. The capsule format makes compliance straightforward, and the oral bioavailability of these ultra-short peptides is well-established. For those already familiar with
injectable versus oral peptide comparisons, bioregulators represent one of the most successful oral peptide formats due to their tiny molecular size.
Starting from age 40-45, Khavinson recommended two full 30-day courses per year. He noted that the health achieved in the decades to follow would be majorly influenced by the degree of recovery accomplished during this critical period of intervention. The biological reserve of the organism can be improved by 42% after a properly cycled bioregulator protocol, a remarkable number that reflects the depth of gene expression restoration achievable with consistent treatment.
Stacking bioregulators
One of the most powerful aspects of the Khavinson system is the ability to stack multiple bioregulators simultaneously. Unlike many peptides where
combining compounds raises interaction concerns, bioregulators are designed to work together. Each targets a specific organ through specific DNA sequences. There is no cross-reactivity or competition between them.
Khavinson recommended stacks of 3-5 bioregulators simultaneously. The research showed that you can safely combine up to 5 peptide complexes at the same time. With 15 million people having undergone peptide treatment over 30 years with no reported side effects, the safety of combining bioregulators is exceptionally well-established. The fact that peptides in animals and humans are identical means the body recognizes them as its own. They normalize protein synthesis without the ability to overstimulate it. This self-limiting mechanism is fundamentally different from hormones or drugs, which can drive processes beyond their optimal range. For anyone building a comprehensive
peptide stack, bioregulators add a gene expression layer that complements surface-receptor peptides. The
stack calculator and
cost calculator at
SeekPeptides can help plan combinations that fit both research goals and budget constraints. Avoiding
common mistakes when starting a multi-compound protocol is always advisable, and the
dosage chart provides a quick reference for standard bioregulator courses alongside traditional peptide protocols.
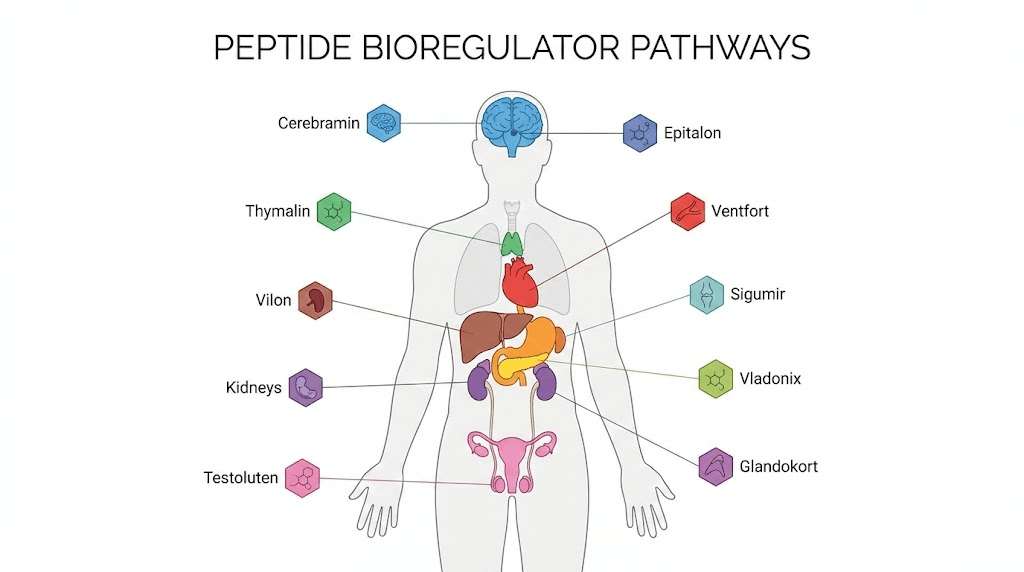
The Khavinson first-class stack
Khavinson himself identified an optimal combination of six bioregulators that he considered the foundation of any comprehensive anti-aging protocol. This is the first-class stack, and it targets the systems that age fastest and have the most systemic impact.
The six bioregulators in the first-class stack are:
- Endoluten (A-8) for the neuroendocrine system (pineal gland)
- Vladonix (A-6) for the immune system (thymus)
- Cerluten (A-5) for the brain and central nervous system
- Sigumir (A-4) for joints and bones
- Svetinorm (A-7) for the liver
- Ventfort (A-3) for blood vessels
Khavinson recommended this stack for one to two months, twice per year. The selection rationale is clear. The pineal gland and thymus are the two master regulators, controlling neuroendocrine and immune function across the entire body. Endoluten and Vladonix are the two most important geroprotectors, capable of extending lifespan by 30-40%. The brain (Cerluten), joints (Sigumir), liver (Svetinorm), and vasculature (Ventfort) represent the organ systems that deteriorate most noticeably with age and have the greatest impact on quality of life. By targeting all six simultaneously, the first-class stack creates a comprehensive restoration effect that addresses aging from multiple directions. Researchers can use the
peptide stack calculator at
SeekPeptides to plan their bioregulator cycles alongside other research compounds.
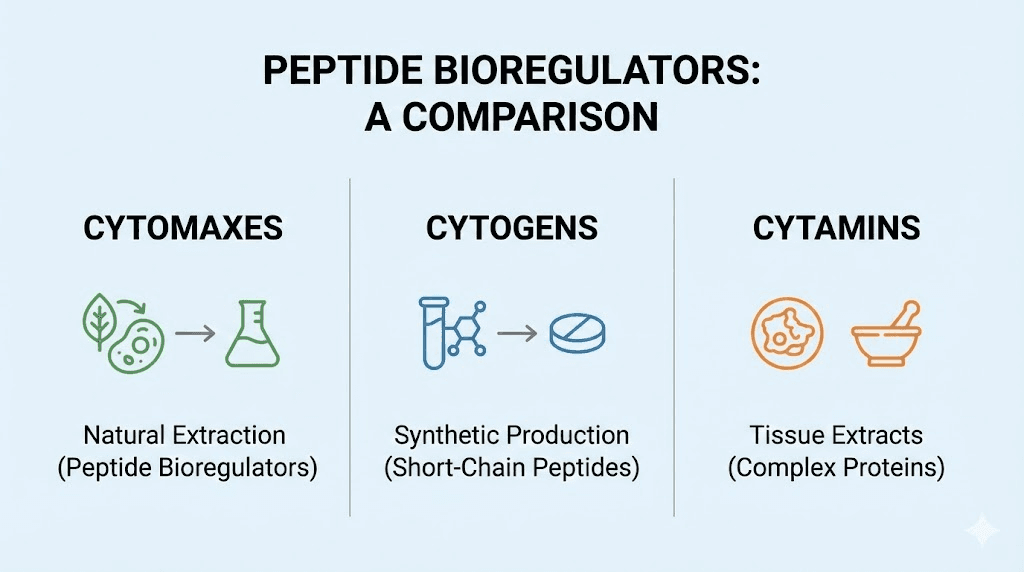
The Cytogens-to-Cytomaxes transition
Experienced bioregulator practitioners follow a specific sequencing strategy that maximizes both speed and depth of therapeutic effect. The protocol begins with Cytogens and transitions to Cytomaxes.
Here is how it works. You start with the synthetic Cytogen version of each target organ bioregulator for the first month. Cytogens have shorter molecules, meaning their action at the initial stage is faster. They kick-start the recovery function immediately, and their effects accumulate 20-30% faster than Cytomaxes. After one month on Cytogens, you transition to the corresponding Cytomaxes for two months. Cytomaxes are approximately 33% stronger and have double the aftereffect duration. They provide the deeper, longer-lasting gene expression restoration.
For example, to support the vascular system, you would start with
Vesugen (Cytogen) for one month, then switch to Ventfort (Cytomax) for two months. For immune support, you would start with Crystagen (Cytogen) for one month, then switch to Vladonix (Cytomax) for two months. This sequential approach maximizes the speed and reduces the cost of the revitalization process. It is important to note that the older the organism, the more conservative it becomes and the less effective Cytogens are alone. For older individuals, repeating Cytogen courses more frequently before moving to Cytomaxes may be advisable. This sequencing approach to
cycling different peptides is unique to the bioregulator system.
For injectable Epitalon specifically, protocols typically use 1-20mg per day for 10-20 day cycles. This injectable route provides more direct systemic access and is preferred by some researchers for the telomerase activation applications. Standard
peptide reconstitution procedures apply when working with injectable formats, and proper
bacteriostatic water is essential. Those using injectable formats should also review the
peptide reconstitution calculator to ensure accurate preparation.
Sublingual dosing and absorption
Beyond capsules and injections, Khavinson bioregulators are also available in sublingual (lingual) preparations. This administration route involves placing 5-6 drops (approximately 0.25-0.35ml) under the tongue for 10-15 minutes before eating, 3-4 times per day for 30 days. The sublingual route offers a significant advantage: absorption occurs immediately through the oral mucosa, bypassing the digestive system entirely. This provides a more direct route to the bloodstream than capsules while avoiding the needle requirements of injectable formats.
The sublingual format also allows for more precise dose adjustment. Unlike capsules, which deliver a fixed dose, lingual preparations can be titrated drop by drop. This fine-tuning capability is particularly valuable for older individuals or those new to bioregulators who want to start conservatively and increase gradually. After a 30-day sublingual course, a 60-day break follows before the cycle repeats. Researchers can use this format every 3-6 months for maintenance or more frequently for therapeutic applications. For a deeper understanding of different peptide delivery methods, the comparison between
injectable versus oral peptides provides additional context, and the
peptide calculator can help with dosing conversions across formats.
Building condition-specific protocols
While the first-class stack addresses general anti-aging, many researchers want to target specific conditions. Khavinson system allows for highly customized protocols built around individual needs. The key is selecting bioregulators that address both the primary organ of concern and the supporting systems that influence its function.
For cognitive decline and brain health, combine Cerluten (brain Cytomax) with
Pinealon (brain Cytogen) and Ventfort (vascular Cytomax). The vascular component is critical because brain function depends entirely on blood supply. Add Endoluten for circadian rhythm support, since poor sleep dramatically accelerates cognitive decline. This protocol addresses neuronal health, neurovascular coupling, and the hormonal environment of the brain simultaneously. Researchers interested in
BDNF-related peptides and
brain function optimization will find that bioregulators complement receptor-based nootropics by addressing the underlying gene expression patterns rather than acute neurotransmitter modulation.
For musculoskeletal issues, combine Sigumir (cartilage and bone Cytomax) with
Cartalax (cartilage Cytogen) and Gotratix (muscle Cytomax). The sequential Cytogen-then-Cytomax approach works particularly well here, starting with Cartalax for rapid cellular activation and transitioning to Sigumir for deep, sustained cartilage regeneration. Adding Ventfort supports the blood supply to recovering tissues. This combination addresses the problem from both the structural tissue level and the vascular supply level. For those already using peptides like
BPC-157 or
TB-500 for acute joint and tendon issues, bioregulators add the long-term gene expression restoration that receptor-based peptides cannot provide. The
wolverine stack concept takes on new dimensions when bioregulators are layered into the healing protocol.
For immune recovery, combine Vladonix (thymus Cytomax) with Crystagen (immune Cytogen) and Endoluten (pineal Cytomax). The thymus and pineal gland work together as master regulators of the immune-neuroendocrine axis. Supporting both simultaneously produces synergistic effects that neither alone can achieve. This was precisely the combination that produced the 4.1-fold mortality reduction in the clinical trial. Those exploring
KPV peptide or other
alpha peptides for immune modulation will find that bioregulators address immune function at a more fundamental level, restoring the thymus capacity to produce naive T-cells rather than simply modulating existing immune responses.
For energy and metabolic support, consider Svetinorm (liver), Suprefort (pancreas), and Endoluten (pineal). The liver drives metabolic function, the pancreas regulates blood sugar and digestive enzymes, and the pineal governs the hormonal rhythms that determine energy cycles throughout the day. Researchers interested in
peptides for energy and
mitochondrial peptides like MOTS-c can layer bioregulators beneath these compounds for complementary gene-expression support. The
NAD peptide pathway also intersects with bioregulator mechanisms at the epigenetic level, and combining both approaches may produce additive benefits.
Safety, side effects, and what to watch for
Safety is the first question any responsible researcher should ask about any compound, and the safety profile of Khavinson peptides is one of their strongest selling points. With over 15 million patients treated across 30 years of clinical use in Russia and Eastern Europe, the accumulated safety data is extensive.
The short answer is that Khavinson peptide bioregulators have an exceptionally high safety profile. No significant toxic, allergic, or adverse effects have been reported across the entire history of their clinical use. This is not surprising when you understand the mechanism. These peptides are identical to sequences naturally present in the human body. The body recognizes them as its own. They normalize protein synthesis but cannot overstimulate it. This self-limiting property is fundamentally different from hormones, drugs, or even larger synthetic peptides that can push biological processes beyond healthy ranges.
That said, some important considerations apply.
Contraindications include individual intolerance to any component, pregnancy, and lactation. Children should consult a healthcare provider before use. Individuals with severely weakened immune systems should proceed with medical guidance. Oral capsule forms have extremely rare side effects. Sublingual preparations occasionally cause mild oral irritation that resolves quickly. Injectable forms like Epitalon carry the standard risks associated with any injection, including injection site reactions, which proper
peptide safety protocols and correct
mixing procedures minimize. The
Biogerontology study on Epitalon provided additional safety reassurance by demonstrating that the peptide activates different pathways in normal versus cancer cells. In normal cells, it upregulates telomerase. In cancer cells, it does not activate telomerase but instead triggers ALT, a fundamentally different mechanism. This selectivity suggests that Epitalon does not promote the kind of uncontrolled telomerase activation that could theoretically fuel cancer growth. Researchers should still approach any longevity compound with appropriate caution, and regular health monitoring is always advisable when using bioregulators or any other
peptide research protocol.
For
storage, capsule-form bioregulators are remarkably stable. They do not require refrigeration in most cases, unlike many research peptides. However, standard best practices apply: store in a cool, dry place away from direct sunlight. Injectable Epitalon follows standard peptide storage guidelines. Understanding
how long peptides last in the fridge and
in powder form helps maintain potency through the full duration of any course. For those concerned about
room temperature stability, bioregulator capsules are among the most resilient peptide formats available. Bioregulators are free from allergens and hormones, and they can be integrated with other supplements and medicines without adverse interactions. This compatibility makes them particularly easy to incorporate into existing
peptide stacks or health regimens.
A common question involves interactions with other health conditions and medications. Because bioregulators normalize gene expression rather than forcing it in one direction, they generally complement rather than conflict with pharmaceutical treatments.
The clinical trial specifically noted that bioregulators were applied in combination with standard therapy for corresponding indications, and the positive outcomes were observed in this combined context. However, individuals with autoimmune conditions should exercise particular caution with thymic bioregulators like Vladonix and Crystagen, as immune activation could theoretically exacerbate autoimmune responses.
This is a theoretical concern rather than an observed clinical problem, but prudent researchers discuss any immune-modulating compound with their healthcare provider before use. For comprehensive guidance on peptide interactions and precautions, the
peptide safety and risks resource covers both bioregulators and traditional compounds.
Those with specific conditions like
fibromyalgia or chronic
pain conditions should consult with practitioners experienced in bioregulator therapy.