I take your points - I've not experienced any issues with the applicator not depressing though.naa if look at actual results they seem pretty consistent. they fudged the data a wee bit as 10000% depends on WHEN they test.. perhaps start to metabolize slower as time goes on? the RANGE looks VERY similar accross those ranges.
testosterone is metabolized very quickly so some people are back to baseline 1 hr after application ie half life of 15 min.. saying median level is super inaccurate as its nothing to do with actual stabilization of free test. just 1 hr after application people range from 400-900 and fairly consistent is all that means. also should not equate that to benefits. ie compare month 1 range to month 6.. pretty much the same -/+ 70 or so....
BE VERY WARY of natesto data... be even more cautious about drawing conclusions from it.
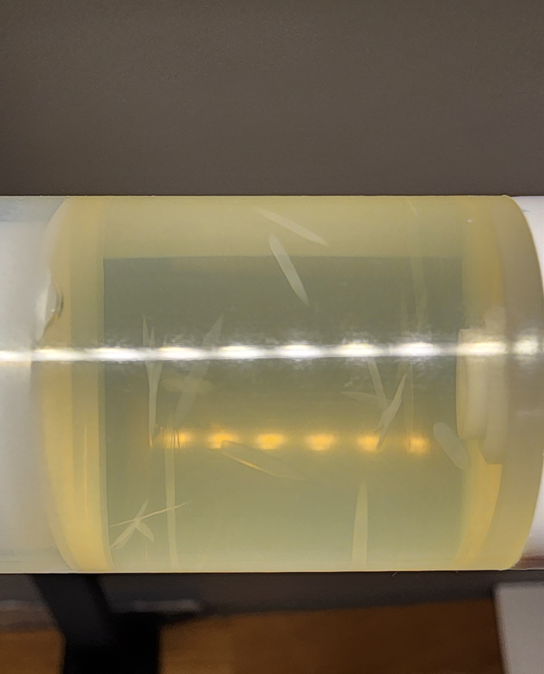
new issue with natesto bottles is the shards get stuck so actuator does not depress all the way... "we will esculate this" yet no refund 7 months later, no answers, empty promises, was told they tested the crystalized product and would send results.... noda... people they had me speak to have left the company lol.. "restructured" have a feeling class action lawsuit is coming and no one wanted to be holding the bag they hired a shell company to manufacture so they dont have to be held accountable.
takes alot of complaints for FDA or health canada to actually do something particularly when no life or death risk as testosterone is pretty safe even if get 4x teh dosage your suppost to AND nearly impossible to gather data unless test 10 min after application AND hit a crystal that exact dose aswell (As 2 pumps 2-3 times a day) as by 1 hr + ur levels will be within range even if were 2500 for a small time. issue is of course causing damage to the HPTA axis having such high unintended spikes.
I may ask the pharmacy to try to exchange the unopened vials from the batch - one of them is riddled with crystals - I'm test/using the one that has the least but its obviously just not an optimal solution and appears to be a widespread manufacturing issue.
Physicians and several pharmacists alike profess total ignorance about this - they claim no one has ever reported it, so I don't know what to think exactly.
Any experience with Empower's 'generic' version of the gel?