madman
Super Moderator
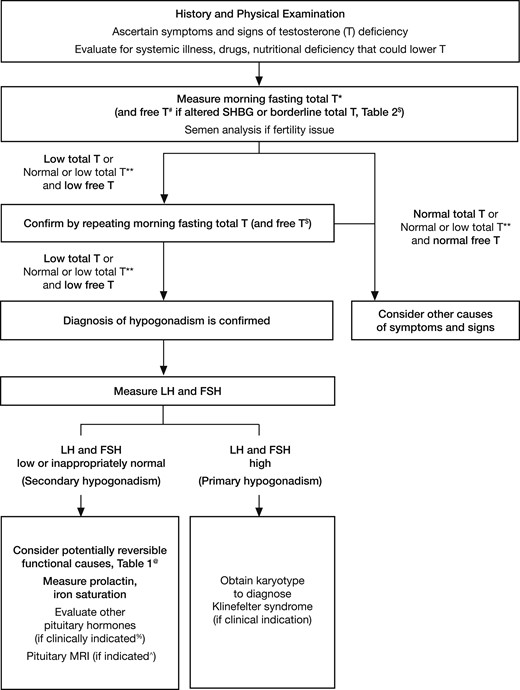
Testosterone Therapy and Men’s Health – Demystifying the Hype with Science to Provide Cutting-Edge Treatment for Your Patients (2021)
Summary 25:10-43:34 (Endocrine Society 2018 Guidelines)

Summary 25:10-43:34 (Endocrine Society 2018 Guidelines)