madman
Super Moderator
IPP Improvements-Recent Innovations, Current Iterations, and Developments in the Pipeline (2022)
Engy Habashy, MD, Raevti Bole, MD, and Sevann Helo, MD
INTRODUCTION
Penile prosthesis surgery is an integral part of the management of erectile dysfunction (ED), Peyronie's disease, and gender affirmation surgery. American Urological Association guidelines support the use of a penile prosthesis in men who have failed or do not wish to pursue nonsurgical treatments.1 Nikolaj A. Bogoraz is credited with the earliest documentation of a penile prosthesis in 1936 using a patient's rib cage and a tubular abdominal flap.2 The first inflatable penile prosthesis (IPP), the cornerstone of modern surgical management of ED, was introduced by Scott and colleagues in 19733,4 Since its inception the IPP has evolved through subsequent iterations to provide a physiologic erection that is natural-appearing in the flaccid and erect states, with the added advantage of being able to generate an erection on-demand for as long as the patient desires.
Currently, 2 companies manufacture IPP devices that are FDA-approved for use in the United States: Boston Scientific (AMS 700, Minnetonka, MN) (Figure 1) and Coloplast (Titan, Humlebaek, Denmark) (Figure 2). Rigicon (Infla10, Ronkonkoma, NY) and Zephyr (ZSI 475, Geneva, Switzerland) also manufacture IPP devices that are currently approved for use outside the United States (Table 1). Competition within the IPP market continues to fuel innovation as manufacturers pursue the goal of prosthesis perfection. Herein we highlight current and forthcoming innovations in IPP technology that aim to improve functionality, durability, as well as patient and partner satisfaction. All manufacturers were contacted for comment on innovations they are actively pursuing, although most were unable to share proprietary information currently under development.
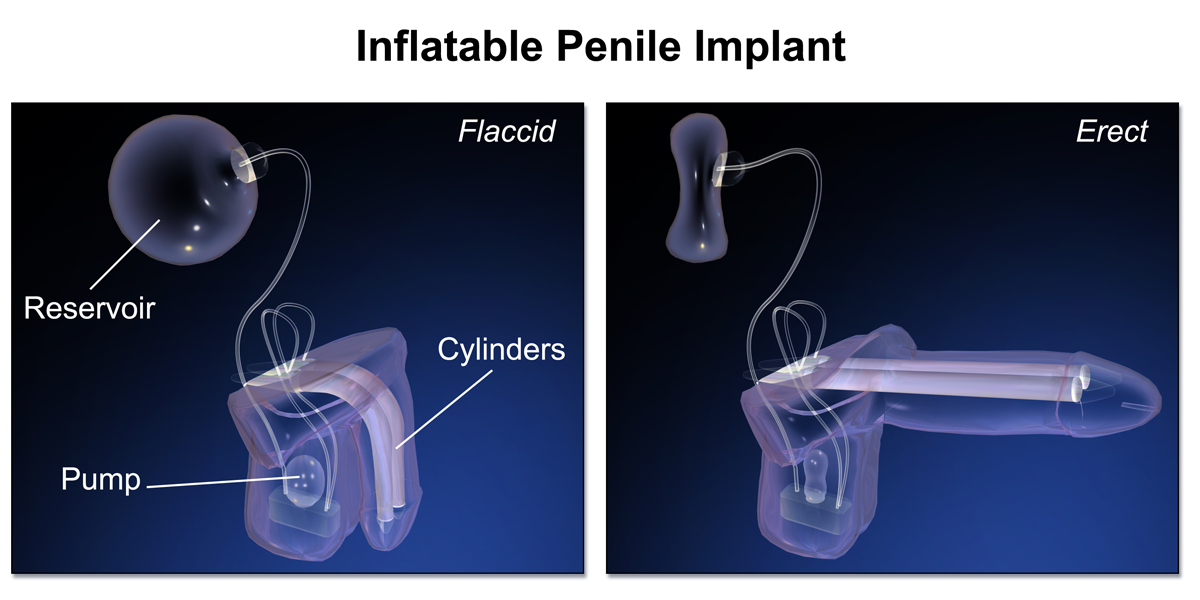
CYLINDERS
The corporal cylinders are arguably the most crucial components of the IPP. They must withstand the repetitive axial force of intercourse with sufficient rigidity while maintaining a low profile in the flaccid state.
PUMP
The IPP pump design is critical as it is the part of the device patients interact with most, and anecdotally the component most likely to generate patient phone calls. It must allow for easy identification as well as facile use of inflation and deflation mechanisms to optimize the utility of the IPP.
RESERVOIR
The reservoir component of a 3-piece inflatable prosthesis stores saline when the cylinders are deflated. The reservoir can be placed via the inguinal ring into the space of Retzius or in a submuscular location depending on patient surgical history, intraoperative findings, and surgeon preference.
ACCESSORIES
Efficient placement of the IPP requires a selection of specialized tools. In the early years, long vascular forceps, snares, and even a cylinder-freezing technique were used to position the device in the corpora.8,9
CONCLUSION
Since its introduction in 1973, the IPP has undergone multiple modifications; each with the goal of improving device function, decreasing complications, and increasing patient and partner satisfaction. The IPP will undoubtedly continue to play an essential role in the treatment of medication-refractory ED. The future for device innovation is bright as more manufacturers join this technologically advanced surgical space and continue to further the evolution of the IPP to optimize the sexual health of cis and transgender patients.
Engy Habashy, MD, Raevti Bole, MD, and Sevann Helo, MD
INTRODUCTION
Penile prosthesis surgery is an integral part of the management of erectile dysfunction (ED), Peyronie's disease, and gender affirmation surgery. American Urological Association guidelines support the use of a penile prosthesis in men who have failed or do not wish to pursue nonsurgical treatments.1 Nikolaj A. Bogoraz is credited with the earliest documentation of a penile prosthesis in 1936 using a patient's rib cage and a tubular abdominal flap.2 The first inflatable penile prosthesis (IPP), the cornerstone of modern surgical management of ED, was introduced by Scott and colleagues in 19733,4 Since its inception the IPP has evolved through subsequent iterations to provide a physiologic erection that is natural-appearing in the flaccid and erect states, with the added advantage of being able to generate an erection on-demand for as long as the patient desires.
Currently, 2 companies manufacture IPP devices that are FDA-approved for use in the United States: Boston Scientific (AMS 700, Minnetonka, MN) (Figure 1) and Coloplast (Titan, Humlebaek, Denmark) (Figure 2). Rigicon (Infla10, Ronkonkoma, NY) and Zephyr (ZSI 475, Geneva, Switzerland) also manufacture IPP devices that are currently approved for use outside the United States (Table 1). Competition within the IPP market continues to fuel innovation as manufacturers pursue the goal of prosthesis perfection. Herein we highlight current and forthcoming innovations in IPP technology that aim to improve functionality, durability, as well as patient and partner satisfaction. All manufacturers were contacted for comment on innovations they are actively pursuing, although most were unable to share proprietary information currently under development.
CYLINDERS
The corporal cylinders are arguably the most crucial components of the IPP. They must withstand the repetitive axial force of intercourse with sufficient rigidity while maintaining a low profile in the flaccid state.
PUMP
The IPP pump design is critical as it is the part of the device patients interact with most, and anecdotally the component most likely to generate patient phone calls. It must allow for easy identification as well as facile use of inflation and deflation mechanisms to optimize the utility of the IPP.
RESERVOIR
The reservoir component of a 3-piece inflatable prosthesis stores saline when the cylinders are deflated. The reservoir can be placed via the inguinal ring into the space of Retzius or in a submuscular location depending on patient surgical history, intraoperative findings, and surgeon preference.
ACCESSORIES
Efficient placement of the IPP requires a selection of specialized tools. In the early years, long vascular forceps, snares, and even a cylinder-freezing technique were used to position the device in the corpora.8,9
CONCLUSION
Since its introduction in 1973, the IPP has undergone multiple modifications; each with the goal of improving device function, decreasing complications, and increasing patient and partner satisfaction. The IPP will undoubtedly continue to play an essential role in the treatment of medication-refractory ED. The future for device innovation is bright as more manufacturers join this technologically advanced surgical space and continue to further the evolution of the IPP to optimize the sexual health of cis and transgender patients.

















