madman
Super Moderator
KNDy Neurons of the Hypothalamus and Their Role in GnRH Pulse Generation: an Update
Abstract. There is considerable evidence that synchronized activity within a reciprocally connected population of cells in the arcuate nucleus (ARC) coexpressin
Abstract
There is considerable evidence that synchronized activity within a reciprocally connected population of cells in the arcuate nucleus (ARC) coexpressing kisspeptin, neurokinin B (NKB), and dynorphin (KNDy cells) is crucial for the generation of gonadotrophin-releasing hormone(GnRH) pulses in mammals. The initial “KNDy hypothesis” proposed that pulsatile GnRH secretion is elicited by episodic kisspeptin release from KNDy cells following synchronized activation and termination of the population by NKB and dynorphin, respectively. Since then, the role of KNDy cells as a critical component of the pulse generator has been further supported by studies at the single-cell level, demonstrating that the population is both necessary and sufficient for pulsatility. In addition, there have been considerable modifications and expansion of the original hypothesis, including work demonstrating the critical role of glutamate in synchronization of the KNDy cell network, functional interactions with other ARC subpopulations, and the existence of species differences in the role of dynorphin in pulse generation. Here we review these recent changes and discuss how the translation of these findings has led to the development of new therapies for disorders related to pulse generation. We also outline critical gaps in knowledge that are currently limiting the application of KNDy research in the clinic, particularly regarding the role of dynorphin in pulse generation in primates.
A History of the Original ‘KNDy Hypothesis’
Since the 1970s, it has been known that the pulsatile release of gonadotrophin-releasing hormone (GnRH) is crucial in the regulation of reproductive function (1). Early work in nonhuman primates pointed to the presence of a pulse generator in the mediobasal hypothalamus responsible for the regulation of episodic GnRH release as well as the subsequent release of luteinizing hormone (LH) from the anterior pituitary (2, 3). Many of the details surrounding the regulation of episodic GnRH release and this pulse generator remain elusive to this day. However, research in the last two decades has focused on hypothalamic kisspeptin-expressing neurons as a central node in the regulation of GnRH/LH release and a key component of the pulse generator (4). This is largely because of the finding that mutations in the gene responsible for encoding the kisspeptin receptor, GPR54, lead to hypogonadotropic hypogonadism both in humans and mice (5, 6). Additional support for kisspeptin’s role as a key component of the pulse generator came from studies showing that the peptide is imperative for various aspects of reproductive function, including its role as a regulator of puberty (7, 8) and estrous cyclicity (9). Kisspeptin directly stimulates GnRH neurons via GPR54, leading to GnRH/LH release (10), and GnRH neuron-specific knockout of GPR54 is associated with fertility (11). Remarkably, the selective expression of GPR54 in GnRH neurons is sufficient to restore puberty, ovarian cycles, and fertility in GPR54 global knockout mice (11).
Traditionally, 2 populations of kisspeptin-expressing neurons have been studied in the context of steroid hormone feedback and GnRH/LH secretion. Although both populations have been shown to express the appropriate receptors for gonadal steroid hormone feedback including estrogen receptor-alpha (ERα) (12), the progesterone receptors (PRs) (13), and the androgen receptor (AR) (14, 15), the kisspeptin-expressing population in the rostral periventricular area of the third ventricle (RP3V) has been closely associated with positive feedback of steroid hormones and surge-like GnRH/LH release(16), while the more caudal population in the arcuate nucleus(ARC) has been associated with negative feedback and GnRH/LH pulse generation (17). This was supported by initial evidence showing that estradiol stimulates RP3V Kiss1 gene expression but inhibits ARC Kiss1 expression (18, 19), as well as recent work showing direct effects of ERα at the level of ARC Kiss1 cells in female mice (20).
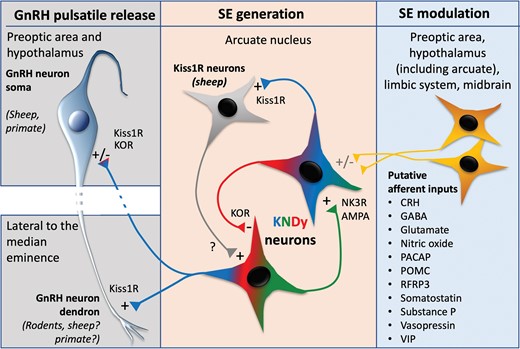
In 2007, multiple-label immunofluorescence studies in the ewe hypothalamus demonstrated that the ARC population of kisspeptin neurons had a high degree of colocalization with the neuropeptides, neurokinin B (NKB), and dynorphin (21). This subpopulation of kisspeptin neurons, referred to as KNDy(kisspeptin, NKB, and dynorphin) cells based on their colocalization of the 3 neuropeptides, was found to be highly conserved across multiple mammalian species, including other ruminants (goat (22)) and in rodents (mouse (23); rat (24)). In contrast, studies of the human hypothalamus (25) suggest that dynorphin may not be coexpressed in kisspeptin/NKB cells of the infundibular nucleus (the human homolog of the ARC), although this is still an unresolved issue (26). Further anatomical evidence revealed that KNDy cells did, in fact, send projections to GnRH neurons both in the preoptic area and mediobasal hypothalamus in sheep (27). This was consistent with previous reports in nonhuman primates (28) and humans (29, 30), but the study in female sheep was unique in that it was the first to show colocalization of multiple KNDy peptides in close contact with the GnRH neurons. By contrast, tract-tracing of KNDy cell projections in rodents identified a lack of innervation to GnRH soma (31). Instead, it was found that KNDy cells exclusively innervate the lateral margins of the median eminence containing distal GnRH dendritic-to-axonal segments (31), termed the dendron (32). As the cell bodies of GnRH neurons are scattered throughout the preoptic area and hypothalamus, the convergence of dendrons in close proximately to KNDy axons provides a site where the episodic release of GnRH by many neurons may be locally controlled via the episodic release of kisspeptin(33, 34).
In addition to their contacts with GnRH neurons, KNDy cells appeared to have reciprocal KNDy-KNDy connections forming an interconnected network capable of producing synchronized firing activity (13, 24). Further studies confirmed the presence of the NKB receptor (NK3R) in KNDy cells in the vast majority of KNDy cells (15, 24, 35-37). Initial in situ hybridization studies in female mice showed low coexpression of Kiss1 and kappa opioid receptor(KOR) messenger RNA (mRNA) in the ARC, with only approximately 20% of Kiss1 cells coexpressing KOR (23). However, later studies using more sensitive in situ hybridization techniques detected 58% and 64% of KNDy cells in female and male mice expressed KOR mRNA, respectively(15), and 29% to 48% of ARC Kiss1R cells coexpressed KOR mRNA in female sheep (36). Importantly, arcuate cells were not found to express GPR54 (38), indicating that KNDy-KNDy signaling is dependent on NKB and dynorphin, while kisspeptin actions occur elsewhere, including GnRH-neurons. GnRH neurons have not been found to express NK3R (35, 39), lending support to the idea that NKB actions occur directly at KNDy neurons. However, studies have shown that KOR is colocalized in GnRH neuron soma in sheep and rats (40), suggesting that dynorphin may act directly at GnRH neurons and indirectly through inhibition of KNDy neurons
Taken together, these findings led to the proposal of the original “KNDy hypothesis.” In brief, this hypothesis stated that NKB acts directly on KNDy cells to synchronize their firing and initiate the onset of a pulse, kisspeptin acts as an output signal to GnRH neurons to stimulate their activity, and dynorphin acts within the KNDy network as a signal to terminate the pulse, stopping both KNDy cell firing and subsequent GnRH/LH release. Since this original hypothesis, substantial progress has been made to both advance (Fig. 1) and challenge various aspects of the proposed mechanism, as detailed in this minireview.
New Evidence that KNDy Cells Comprise a Core Element of the Gonadotrophin-Releasing Hormone/Luteinizing Hormone Pulse Generator
KNDy Cells Are Necessary and Sufficient for Luteinizing Hormone Pulses
The aforementioned foundational studies demonstrate that KNDy cells contain auto-excitatory and autoinhibitory components capable of generating episodic, synchronized activity that drives GnRH/LH pulses. Consistent with this, cell-specific activation of KNDy cells using brief optogenetic stimulation is sufficient to generate an LH pulse in mice (41, 42). Conversely, optogenetic inhibition of endogenous KNDy cell activity reduces the frequency and amplitude of LH pulses (43). This is comparable with recent findings in sheep, in which a reduction in LH pulse amplitude and frequency was seen after lesioning 90% of the KNDy cell population (36). Although these studies did not observe the complete elimination of pulses, this is likely due to incomplete inhibition or lesioning of the entire population. The idea that only a portion of kisspeptin cells is required for reproductive capacity has been previously supported by studies in which mice with kisspeptin levels were genetically reduced still exhibit puberty, estrous cycles, and fertility (44). Further, a recent study using kisspeptin knockout rats, which exhibit no gonadotrophin secretion, had LH pulses restored with the reintroduction of kisspeptin to only 20% of ARCNKB (KNDy) cells (45). Therefore, kisspeptin release from only a limited subset of KNDy cells is sufficient to generate detectable LH pulses.
Episodic KNDy Cell Activity Is Coupled With Luteinizing Hormone Pulses
The KNDy hypothesis requires evidence that synchronized and episodic activity among KNDy cells triggers GnRH and LH pulses. A caveat of the studies reviewed earlier is that the large-scale manipulation of cells may obscure details regarding potentially complex and dynamic patterns of activity that occur in situ to control LH pulse secretion. More recently, techniques have been used to monitor KNDy cell activity, including at the single-cell level, in freely behaving mice. Although recording the in situ activity of arcuate populations has been historically challenging due to the deep location of this nucleus in the brain and the number of molecularly and functionally distinct cells within it, the recent ability to record intracellular calcium levels, a proxy for cell activity, in genetically defined cells of freely moving transgenic mice has enabled direct correlation of KNDy cell activity and pulsatile LH secretion. In landmark experiments, in vivo, calcium recordings of KNDy cells in mice using fiber photometry revealed near-perfect episodic activity of KNDy cells preceding an LH pulse (45). A limitation of fiber photometry is its inability to differentiate changes in calcium signals between individual cells, which restricts the detection of anatomically and functionally distinct subpopulations. The implantation of gradient refractive index (GRIN) lenses coupled with miniature microscopy to record intracellular calcium levels in individual KNDy cells was achieved only within the past year, unveiling synchronized episodes (SEs) of activity among recorded cells that occurred episodically in gonadectomized female (46) and male (47) mice. The study by Moore et al (46) revealed that LH pulses were nearly always preceded by SEs in which all recorded cells were activated. Further, cell activity was observed to start in a subset of cells and propagate through the remaining population in a predictable temporal order. These findings suggest the existence of “leader cells” that initiate robust SEs capable of driving LH pulses, although the molecular components by which leader KNDy cells initiate episodic activity remain to be revealed. In contrast, a similar study by Han et al in male mice (47) reported a much looser degree of temporal ordering. This may be due to sex differences between female and male mice or differences in the number of SEs obtained in a recording between studies, as the fewer SEs recorded in male mice may diminish the ability to detect temporal patterns. It was proposed that the handling of mice by investigators during blood sampling may “pace” the generator and drive temporal ordering; however, data from our laboratory show distinct populations of leader and follower cells even when ovariectomized mice remain unhandled (Moore et al unpublished data). Regardless, all reported studies using in vivo recording of KNDy cells have concluded that the release of LH tightly corresponds with synchronized KNDy cell activity, supporting the hypothesis that these cells form a crucial component of the GnRH pulse generator. On a further note, there is evidence to suggest that KNDy cells may also play a role in generating the preovulatory GnRH surge in ruminants. In ewes, during the surge, KNDy neuron colocalization of the immediate early gene cFos increases (48-51), indicating higher activity in these cells, which may contribute to a kisspeptin-mediated increase in the amplitude of the GnRH surge (52). The mechanisms through which KNDy cells mediate pulsatile vs surge modes of secretion are not yet well understood. However, serial blood sampling from portal vasculature in ewes has shown that the surge is composed of GnRH pulses that continue with increasing frequency on a background of elevated basal GnRH (53). Further research is required to determine the contribution of rapid GnRH pulse frequency to surge generation and whether subpopulations of KNDy cells are responsible for pulse and surge modes of secretion.
Kisspeptin Released by KNDy Cells Is Required for Pulsatile Gonadotrophin-Releasing Hormone Release
According to the KNDy hypothesis, kisspeptin release from KNDy cells serves as the output driving GnRH and gonadotrophin release. As a prediction of this hypothesis, eliminating ARC kisspeptin should either reduce the amplitude of pulses or eliminate them altogether. Consistent with this, the knockout of Kiss1 from rats (45, 54, 55) results in absent LH pulses. When kisspeptin is restored to subpopulations of KNDy cells, the amplitude of pulses is significantly greater in rats that express kisspeptin in more than 20% of neurons compared to those with more moderate expression (3%-15%) (45). These data support a direct role for kisspeptin serving as the stimulatory output driving pulses. Surprisingly, LH pulses continue when kisspeptin is selectively knocked out from KNDy cells in mice, although at a reduced frequency and amplitude (56). Although it is unclear why pulses continue in this model, it may indicate compensatory or redundant mechanisms involving other neural populations that are recruited to maintain an excitatory output to GnRH neurons in the absence of kisspeptin in mice. To avoid the risk of developmental compensation, viral-mediated knockdown approaches have been used to assess the effect of diminished kisspeptin in KNDy cells. Reducing the expression of ARC kisspeptin in adult rats (57) and mice (58) was found to reduce LH pulse frequency, but not amplitude. It is unclear how a decrease in kisspeptin alters pulse frequency in these models, but may indicate that kisspeptin is involved in regulating the timing of the GnRH pulse generator (discussed later).
Neurokinin B Synchronizes KNDy Cell Activity
The ability of NKB-NK3R signaling to induce LH release has been supported by data in which the infusion of NKB or senktide, an agonist of NK3R, into the lateral ventricle or ARC of ruminants (goat (22, 59, 60); sheep (61)); and mice (35) elicits LH release. It is unlikely that NKB directly stimulates GnRH/LH secretion as GnRH neuron cell bodies do not express NK3R in the sheep (39) or rodent (62, 63), although colocalization with axon terminals has been reported in the rat (63). Instead, NKB release from KNDy cells has been hypothesized to activate and synchronize reciprocally connected KNDy cells to elicit episodic kisspeptin release onto GnRH neurons. In goats, multi-unit activity volleys in the ARC correlate with senktide-induced release of LH, supporting the effect is mediated by an NK3R-expressing ARC population such as KNDy cells (22). This was directly tested using single-cell recordings in the mouse brain slice, in which NKB and senktide depolarize KNDy cells (37, 64). Although the effects of senktide can be blocked by an NK3R antagonist (64), NKB-induced depolarization was only blocked using antagonists for all 3 tachykinin receptors, suggesting that redundant signaling mechanisms exist in mice (64). In a later study, the antagonism of NK3R alone was sufficient to block the induction of slow excitatory postsynaptic potentials in KNDy neurons that were induced by NKB release from optogenetically stimulated KNDy cells (65). This study further suggested that NKB-NK3R signaling occurred via KNDy-to-KNDy synaptic connections. Therefore, there is strong support that NKB can activate KNDy cells via reciprocal connections to synchronize the population. In a more recent study using calcium imaging, NKB initiated episodes of KNDy cell activity in the mouse brain slice and increased the frequency of events and the number of cells synchronized (47). However, AMPA receptor antagonism reduced SE frequency in the presence of NKB, suggesting a role for glutamate in initiating SEs (discussed further later). This may reflect considerable species differences in the role of NKB, as tachykinin antagonism does not alter KNDy cell SE in mice (47) or LH pulse generation in rats (66), but the intra-ARC antagonism of NK3Rslows LH pulse generation in sheep (61).
Role of Dynorphin
There is strong support from studies in ruminants (goats and sheep) that dynorphin acts as a stop signal terminating GnRH/LH pulses, as the KOR antagonist nor-BNI increases LH pulse frequency (61) as well as multi-unit activity corresponding to pulses (22). There is also strong evidence in sheep that dynorphin acts as an inhibitory signal and plays a critical role in mediating the suppressive effects of progesterone on LH pulse frequency (67). In contrast, while a majority of kisspeptin +NKB cells in mice express dynorphin, it does not appear to play a significant endogenous role in pulse inhibition in this species. Nor-BNI can depolarize KNDy cells in brain slices after stimulation with optogenetics (65) or block the senktide-induced suppression of LH pulses (61); however, the drug has no effect on the basal activity of KNDy cells in slices (37) or on the endogenously driven pulsatile secretion of LH in vivo in rodents (66). Thus, while delivery of KOR antagonists can alter KNDy cell activity under conditions in which they are driven by exogenous stimuli, endogenous dynorphin does not appear to play a physiological role in LH pulsatility. Consistent with this, LH pulses are unaffected by the cell-specific knockout of KOR from mouse kisspeptin cells(68). However, recent in vivo calcium imaging work suggests that dynorphin may play a modulatory gating role in facilitating glutamate-driven synchronization of KNDy neurons in male mice (47). The role of dynorphin in primates, including humans, remains unclear. While the nonspecific opioid antagonist, naloxone, increases LH pulse frequency during the late-follicular and luteal phase of the human menstrual cycle, the effects of nor-BNI on LH pulses in humans or nonhuman primates have not been explored. Dynorphin expression is seen in the infundibular nucleus of nonhuman primates but only within a very small percentage of kisspeptin cells (69); the same low percentage of colocalization is seen in young men (25) and postmenopausal women (70). Therefore, the possible role of dynorphin in pulse generation in primates remains unresolved and is an important area for future study.
Expansion and Modification of the KNDy Cell Hypothesis
While studies over the past decade have provided support for the roles of the original components of the KNDy hypothesis, recent work has expanded the hypothesis in several key aspects. First, there is now compelling evidence that glutamate, coexpressed in KNDy cells, serves as an obligatory driver of synchronized activity among the population. Initial evidence in mice showed that blockade of glutamatergic AMPA and N-methyl-D-aspartate (NMDA) receptors in vivo markedly reduced LH pulse frequency (71), and subsequent work in arcuate slices using calcium imaging to detect synchronized KNDy neuron events showed the same ability of AMPA antagonists, alone or in combination with NMDA antagonists (47). The striking ability of glutamate antagonism to block pulsatile activity suggested the idea that the level of network excitability is critical for pulse generation. Additional observations from in vitro calcium imaging studies furthermore suggested a more nuanced view of the role of NKB and dynorphin in pulse generation, with NKB potentiating glutamate-driven synchronization and dynorphin blocking the initiation of that synchronization (47). In sheep, NMDA receptor antagonist delivery into the ARC had no effect on LH pulses (61), although AMPA antagonists have yet to be tested. Nor is there direct evidence bearing on the role of AMPA receptors in pulse generation in nonhuman primates, although push-pull perfusates of third-ventricle cerebrospinal fluid have shown that LH pulses are always preceded by increases in glutamate release but not that of γ-aminobutyric acid (72).
Another major change in the conceptualization of the KNDy pulse generator is evidence for the role of adjacent, non-KNDy neurons of the ARC in this circuitry, specifically neurons that express the kisspeptin receptor, Kiss1r. Initial evidence of the role of ARC Kiss1r cell came from studies in rats (73) and sheep(61), showing the ability of kisspeptin antagonists microinjected into the ARC to decrease LH pulse frequency. More recently, this evidence was extended by the use of cell-specific lesions to eliminate Kiss1r cells restricted to the ARC (36). Peptide conjugates of the ribosome-inactivating protein, saporin, were used to selectively lesion either KNDy or Kiss1r-containing cells, and the elimination of one but not the other population was carefully confirmed with multiple label in situ hybridization. Importantly, Kiss1r lesions eliminated cells only in the ARC and had no effect on either GnRH cell bodies or their fibers in the nearby median eminence. Whereas KNDy cell lesions resulted in an almost complete elimination of LH pulses, lesions that ablated 87% to 100% of ARC Kiss1r cells had no effect on LH pulse frequency but markedly reduced pulse amplitude and, more strikingly, increased variability in the timing of pulses (36). This evidence suggests that ARC Kiss1r neurons in sheep may form a positive feedback loop with the KNDy circuitry that reinforces synchronized neural activity and the timing of pulses. However, this role of Kiss1r cells is likely not present in mice since selective reinsertion of Kiss1r only in GnRH neurons is sufficient to restore fertility in mice with global knockout of Kiss1r (11). Furthermore, selective knockout of kisspeptin in KNDy neurons did not affect synchronized activity patterns of their activity in vivo that normally correlate with LH pulses(33). Whether Kiss1r neurons of the primate infundibular nucleus play any role in pulse generation is unknown, but it is interesting to speculate that a role of these cells may provide an explanation for the ability of kisspeptin to induce LH pulses in humans with inactivating mutations in NKB or its receptor (74).
Clinical Application of the KNDy Cell Hypothesis
In a remarkably short period of time since discovering the role of KNDy peptides in reproduction and pulse generation, kisspeptin and NKB have been recognized as viable targets for diagnosing and/or treating a wide range of disorders in the reproductive endocrine axis. For instance, circulating levels of kisspeptin may act as a tool for the differentiation and diagnosis of constitutional delays of growth and puberty, congenital hypogonadotropic hypogonadism (75), precocious puberty(76), hypothalamic amenorrhea (77-79), and pregnancy complications (80-83). The ability of kisspeptin to induce GnRH release has been extensively studied to treat disorders with low gonadotrophin secretion, such as hypothalamic amenorrhea (79) or hyperprolactinemia (84-86). Further, NK3R antagonists have emerged as a promising tool to treat syndromes that require suppression (but not elimination) of GnRH pulse generation and steroid hormone secretion, such as polycystic ovary syndrome (87) and uterine fibroids (88). Although many of the aforementioned studies remain in clinical trial stages, recently, the US Food and Drug Administration approved once-daily administration of the NK3R antagonist fezolinetant to reduce the frequency and severity of hot flash symptoms in postmenopausal patients (89, 90). This likely acts by antagonizing KNDy innervation of NK3R receptor-expressing cells in the median preoptic nucleus that normally mediate hormonal regulation of body temperature regulation (91).
Conclusions and Future Directions
A convincing body of evidence supports the view that episodic synchronized activity in KNDy cells is critical for pulse generation across mammalian species. Along with this, evidence continues to support the roles of kisspeptin and NKB as stimulatory peptides in the original KNDy hypothesis; nonetheless, NKB’s role may be more nuanced than originally envisioned and depend on interactions with network excitability mediated by glutamate and depend on the steroidal milieu. By contrast, evidence for a role of dynorphin in pulse generation varies widely between species: In ruminants (sheep and goats), dynorphin plays a clear inhibitory role in modulating GnRH pulses, whereas in rodents, although the intrinsic machinery consisting of the peptide and its receptor are present, a physiological role for dynorphin in pulse generation is questionable. Among recent additions to the original KNDy hypothesis is the critical role of glutamate in synchronization of KNDy cells, mediated primarily by AMPA receptors. In addition, evidence in sheep and rats suggests that adjacent Kiss1R cells in the ARC may form a positive feedback loop with KNDy cells regulating the regularity of the timing of pulses, although it does not appear to play this role in mice. Although not covered in detail in this review, there is also substantial evidence for other cell populations that are afferent to KNDy neurons (see Fig. 1) playing a role in modulating their activity, although in most cases their precise role in pulse generation remains to be explored.
As noted earlier, perhaps the most striking aspect of the development of the KNDy hypothesis has been the relatively rapid translation from discovery of this neuronal population to the application of drugs that act on KNDy peptide receptors in the treatment of clinical disorders affecting reproduction as well as other functions (92). As noted previously, these include the recent Food and Drug Administration approval of the NKB receptor antagonist, fezolinetant, in the treatment of hot flashes (89, 90) as well as studies demonstrating the efficacy of NKB antagonists in reducing LH pulse frequency in PCOS (87). Nonetheless, in light of this relatively rapid translation from basic knowledge to clinical practice, a major missing piece of evidence regards the role of KNDy peptides and neurotransmitters in pulse generation in primates. In particular, the role of dynorphin in pulse generation in primates remains largely unexplored (26); the low coexpression of dynorphin in kisspeptin- or NKB-containing neurons argues against a role (25, 69), but the ability of the nonspecific opioid antagonist, naloxone, to increase pulsatile LH secretion(93-95) is consistent with it. Similarly, the physiological role of glutamate release in pulse generation in primates, and the corresponding roles of AMPA and/or NMDA receptors at the level of the ARC, are areas to be explored. Finally, the ability to ascribe the roles of KNDy peptides and neurotransmitters to actions directly on neurons of the ARC, or whether other regions/afferents are involved, will depend on work in nonhuman primates and/or other models in which sites of action can be experimentally restricted. Given recent findings demonstrating extra neuroendocrine roles for kisspeptin in sexual libido (96) and possibly other functions, it may be that KNDy neurons and their unique cohort of coexpressed neuropeptides also have functional roles outside the neuroendocrine system that remain to be revealed.

















