Frontiers | Re-Expression of ERα and AR in Receptor Negative Endocrine Cancers via GSK3 Inhibition
DNA methylation, catalyzed by DNA methyltransferase (DNMT), is a well-characterized epigenetic modification in cancer cells. In particular, promoter hypermet...
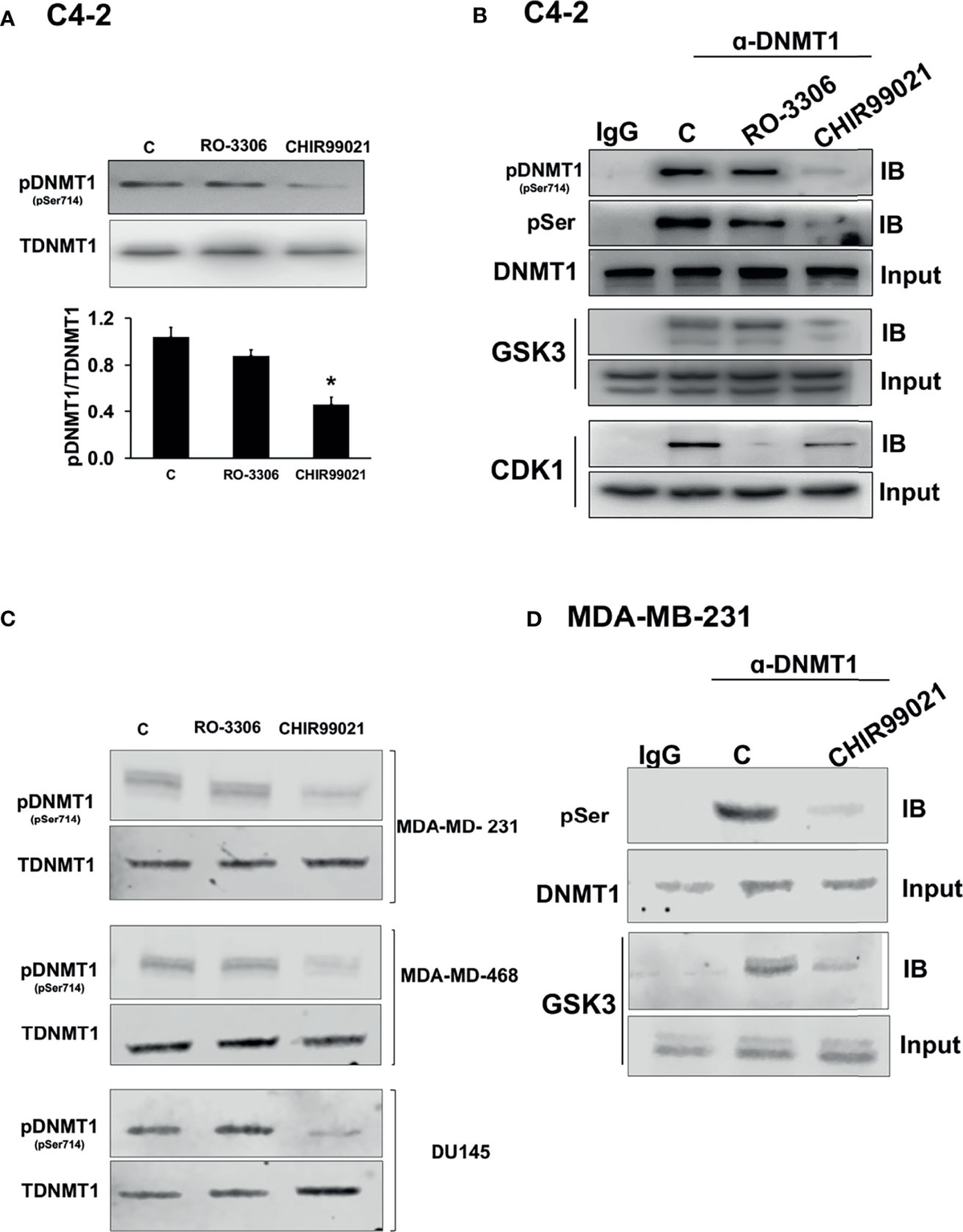
This is the most important study i could find
Lithium is a GSK-3 Inhibitor, but weak, Lithium Ascorbate is the best lithium salt for this, because of several reasons, but likely still too weak. Tideglusib seems like the best drug available to us to re-express the AR and ER in cells in which these receptors got silenced by DNA methylation. Meso tried to achieve unsilencing with HDACi/DNMTi but this seems like the far superior option
Tideglusib has almost no side effects
Running it at 1g/day soon, i will let you know if it works
1. "Evidence that AR/ER down-regulation is a causal mechanism in PSSD. We just do not know."
Assessment: True (but with emerging convergent evidence supporting plausibility). This statement holds because no direct, causal human studies (e.g., CNS tissue biopsies or longitudinal AR/ER expression tracking pre/post-SSRI) exist for PSSD specifically. Causality requires ruling out confounders like underlying depression or 5-HT1A receptor changes, and ethical barriers limit invasive CNS research. However, it's not a complete void—preclinical and proxy human data suggest AR/ER silencing via epigenetic mechanisms (e.g., promoter hypermethylation) as a plausible contributor, mirroring post-finasteride syndrome (PFS).
2. "Evidence that GSK-3 inhibition in the right tissues in humans will restore AR/ER function."
Assessment: Partially true (strong preclinical support for the mechanism, but human CNS-specific restoration data is indirect and extrapolated). True in the strict sense—no dedicated human trials measure AR/ER re-expression post-GSK-3 inhibition in PSSD or similar syndromes. GSK-3β phosphorylates DNMT1 (DNA methyltransferase 1), promoting AR/ER promoter methylation; inhibition should demethylate and restore expression. But human evidence is from AD/PSP trials, not sexual dysfunction models, and focuses on tau/β-catenin, not steroid receptors.
3. "The many off-target effects which might be dangerous."
Assessment: Partially true (off-targets exist theoretically, but clinical data shows a favorable short-term profile; "many" and "dangerous" overstate based on trials). True that GSK-3β inhibition has broad effects (e.g., Wnt/β-catenin hyperactivation risking tumorigenesis; non-selective kinase hits), but Tideglusib's profile in Phase II trials (n>300, 400–1000 mg/day, up to 52 weeks) is mild, with no serious adverse events (SAEs) and low discontinuation (35%, mostly GI). Long-term (>1 year) risks are untested in non-AD populations like young PSSD patients.
4. "Evidence that the downstream sexual/neurological effects of receptor re-expression translate into symptomatic improvement."
Assessment: True (direct evidence is absent; only indirect/anecdotal signals exist). No RCTs link AR/ER re-expression to PSSD symptom relief—PSSD trials are scarce, and GSK-3i endpoints don't include IIEF/ASEX scales. However, related syndromes (e.g., PFS) and proxies suggest potential: Re-expression might restore libido/sensation via hypothalamic rewiring, but lags (months) and variability (e.g., 5-HT1A overlap) could blunt gains.












