madman
Super Moderator
On March 23, 2020, an approved application for a biological product under section 505 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) was deemed to be a license for the biological product under section 351 of the Public Health Service Act (PHS Act) (see section 7002(e)(4)(A) of the Biologics Price Competition and Innovation Act of 2009).
To enhance transparency and facilitate planning for March 23, 2020, transition date, FDA compiled a preliminary list of approved applications for biological products under the FD&C Act that were listed in FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book) and that would be affected by this transition provision. FDA posted this list on the FDA website in December 2018, and periodically updated this list before the March 23, 2020, transition date. The September 2019 update to this preliminary list added certain administratively closed applications related to approved applications for biological products that were on the December 2018 version of this list. The January 2020 update to the preliminary list reflected a change to the definition of “biological product” made by the Further Consolidated Appropriations Act, 2020, which was enacted on December 20, 2019. Section 605 of this Act further amended the definition of a “biological product” in section 351(i) of the PHS Act to remove the parenthetical “(except any chemically synthesized polypeptide)” from the statutory category of “protein.”
FDA has provided below a list of each approved application for a biological product under the FD&C Act that was deemed to be a license (i.e., an approved biologics license application (BLA)) for the biological product on March 23, 2020. These former NDAs have been removed from FDA’s Orange Book and were added to FDA’s “Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations” on March 23, 2020. On April 2, 2020, FDA updated this list to correct an error in the listing of the names of two application holders.
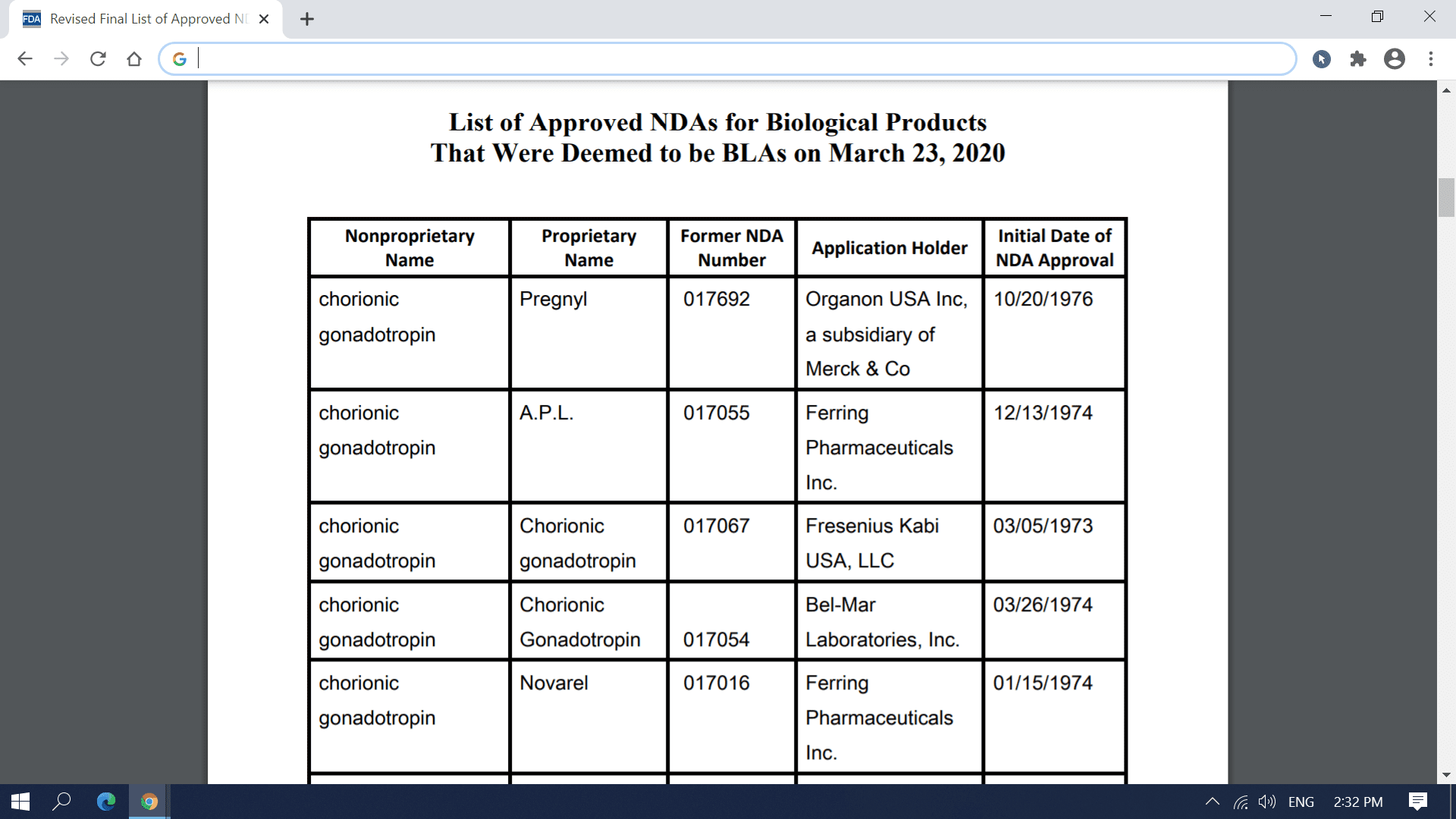
To enhance transparency and facilitate planning for March 23, 2020, transition date, FDA compiled a preliminary list of approved applications for biological products under the FD&C Act that were listed in FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book) and that would be affected by this transition provision. FDA posted this list on the FDA website in December 2018, and periodically updated this list before the March 23, 2020, transition date. The September 2019 update to this preliminary list added certain administratively closed applications related to approved applications for biological products that were on the December 2018 version of this list. The January 2020 update to the preliminary list reflected a change to the definition of “biological product” made by the Further Consolidated Appropriations Act, 2020, which was enacted on December 20, 2019. Section 605 of this Act further amended the definition of a “biological product” in section 351(i) of the PHS Act to remove the parenthetical “(except any chemically synthesized polypeptide)” from the statutory category of “protein.”
FDA has provided below a list of each approved application for a biological product under the FD&C Act that was deemed to be a license (i.e., an approved biologics license application (BLA)) for the biological product on March 23, 2020. These former NDAs have been removed from FDA’s Orange Book and were added to FDA’s “Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations” on March 23, 2020. On April 2, 2020, FDA updated this list to correct an error in the listing of the names of two application holders.

















