T
tareload
Guest

Pregnancy disrupts the accuracy of automated fT4 immunoassays
Objective Thyroid hormone measurements are often performed in pregnant women, as hypo- and hyperthyroidism during pregnancy can severely affect the fetus. Serum free thyroxine (fT4) measurements are well known for their analytical challenges, due to low serum concentrations and the subtle...

Are we having fun yet?
Discussion
In this study, we performed a method comparison between five automated commercially available fT4 IAs routinely used in clinical laboratories and an fT4 (candidate) reference method (equilibrium dialysis combined with LC-MS/MS) in pregnant women and healthy controls to evaluate whether fT4 IAs measure accurately in serum of pregnant women. Our results indicated that fT4 concentrations in pregnant women measured using IAs were often falsely increased leading to a potential overestimation of thyroid hormone status. Results will be discussed below.
We showed that fT4 concentrations measured using both LC-MS/MS and IAs were significantly lower in the samples of pregnant women compared to the samples of healthy controls, which is supported by previous literature (22, 23). In a healthy, iodine-sufficient pregnant population, this is an observation considered normal physiology and not the result of a technical artifact of the measurement method. Although there has been no definite explanation for why serum fT4 concentration is decreased during pregnancy, several mechanisms are suggested to underlie this observation. HCG increases estrogen concentrations during pregnancy and consequently, estrogen increases TBG concentrations causing more T4 to bind to TBG which might lead to short-term lower freely circulating T4 (24). On the other hand, albumin concentrations decrease during pregnancy because of a dilution effect caused by an increased total blood volume (25). Although the changes in binding protein concentration will alter the total amount of bound T4, the fT4 concentration is dependent upon feedback from the pituitary by thyroid-stimulating hormone (TSH). A short-term inappropriately decreased fT4 will be sensed by the pituitary and lead to an increased TSH and stimulation of the thyroid gland to produce T4 which will ultimately result in an fT4 suitable for normal physiology during pregnancy. As another explanation, it is suggested that hemodynamic changes (e.g. increase of total blood volume) during pregnancy may lead to a lower fT4 concentration because of a dilution effect. In addition, increased type 3 deiodinase activity in the placenta is suggested to cause increased consumption of maternal thyroid hormones as well (26). On the other hand, it has also been proposed that aforementioned factors are not the cause of decreased fT4 concentrations but that the lower fT4 concentrations during pregnancy resemble the changes seen during non-thyroidal illness (NTI) (27). The observed decrease in fT4 concentration during pregnancy is, however, not worrisome because of an adaption of thyroid hormone-responsive cells by increasing the capacity of nuclear receptors (28). Such adaption can compensate for the lower fT4 concentrations and indicates altered thyroid homeostasis during pregnancy.
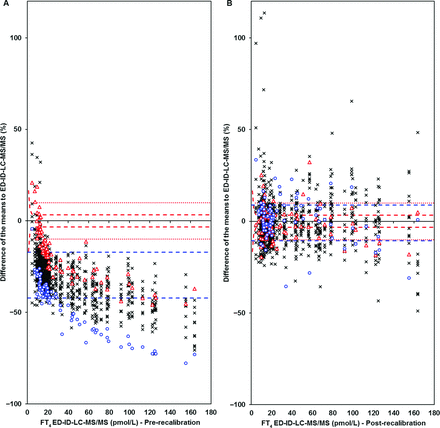
Secondly, we showed that all tested IAs measured lower fT4 concentrations compared to the LC-MS/MS in healthy controls, indicating a systematic bias which has been described previously (19). Indeed the International Federation of Clinical Chemistry working group for Standardization of Thyroid Function Tests acknowledged the need for standardization of the fT4 assay. Standardization will improve the comparability of the fT4 assays enabling worldwide generalized reference intervals, will improve interpretation and will prevent miscommunication regarding fT4 results. However, there are other methodological quality aspects (like matrix effects in a pregnant population as demonstrated in this study) that need to be addressed in addition to implementation of standardization (29). Although less than in healthy controls, most IAs measured significantly lower fT4 concentrations in serum of pregnant women compared to the LC-MS/MS as well. Atellica and Cobas IAs, however, did not measure significantly lower. As will be discussed in detail below, the Atellica and Cobas IAs showed the largest positive bias in serum of pregnant women. This explains the fT4 concentration measured using these IAs was (falsely) higher and reached a comparable level in pregnant women to the concentrations measured by the LC-MS/MS. This example shows the complexity of the process of fT4 standardization for all types of patients and their specific matrices.
Correction of a systematic bias by standardization may improve the comparability of the fT4 assays in healthy controls yet will not directly lead to accurate IA measurements in pregnant women. Furthermore, even though fT4 concentrations measured using both IAs and the LC-MS/MS are lower during pregnancy, the degree of this decrease is method-dependent in the immunoassays which can, thus, be attributed to methodical differences. Our study showed that in all tested IAs, fT4 concentrations in samples of pregnant women deviated positively from the LC-MS/MS method (+7.2 to +28.7%), meaning that all IAs overestimate the thyroid hormone status in pregnant women. The degree to which IAs differ is consistent with literature (12, 30, 31). Nonetheless, our study is unique in showing these results with multiple IAs currently and frequently used in clinical laboratories set against a (candidate) reference method. Although TSH within the reference interval will most likely not be followed by the measurement of fT4, the overestimation of fT4 in pregnant women could especially have consequences if TSH is deviant and fT4 is around the lower or upper limits of the reference interval if these reference intervals are not adjusted correctly for pregnancy. To illustrate, fT4 concentrations in pregnant women around the lower limit but within the reference interval of healthy controls measured using an IA may be below the reference limit when measured using LC-MS/MS. Even if standardization of the IA toward the LC-MS/MS for healthy controls has been performed, this does not solve the deviation between pregnant women and healthy controls and may lead to under diagnosis of maternal hypothyroidism. In most cases, TSH is measured first and will be increased in case of (subclinical) hypothyroidism, even though fT4 may not be lowered (yet). The distinction between subclinical hypothyroidism and overt hypothyroidism can easily be missed if fT4 is erroneously within the reference intervals. Subclinical hypothyroidism in pregnancy is treated with levothyroxine only under specific conditions, whereas it is strongly recommended to treat overt hypothyroidism in pregnancy with levothyroxine (32). This clear difference in treatment strategy indicates the relevance of our findings. Our study was not designed to determine the extent to which this phenomenon causes problems in clinical practice, so more research is needed. At the upper limits of the reference interval, an overestimation of thyroid function could have opposite consequences; pregnant women could be suspected of hyperthyroidism due to erroneously increased fT4 concentrations. However, the latter will probably have less impact because TSH is most likely not deviant in this case.
Previous literature suggested that alterations in TBG and albumin could cause fT4 assay inaccuracies (11, 12, 33, 34, 35), whereas it is most likely that the fT4 LC-MS/MS reference method does not show protein-binding-dependent aberrations due to strict adherence to the defined conventions (20) (Jansen et al. in preparation). Our results confirmed that TBG concentrations were significantly higher and albumin concentrations were significantly lower in samples of pregnant women compared to healthy controls (36, 37). However, the influence of albumin on fT4 assay accuracy during pregnancy has only been investigated using RIAs which cannot directly be extrapolated to automated IAs (13, 14). The influence of altered concentrations of TBG on IA measurements has been better established, although its influence during pregnancy is less evident (12, 33). Combined results of ours and previous studies showed that TBG and albumin concentrations may play a role in the fT4 IA bias in pregnant women, although a causal correlation cannot be confirmed (11, 38). Since TBG concentrations may play a role in fT4 IA inaccuracies, this might have consequences in other specific groups characterized by increased TBG concentrations, such as women using oral contraceptives or using estrogens after menopause, as well. Clearly, more research is needed to investigate other specific groups.
Our results indicate problems in the accuracy of measuring fT4 concentrations in pregnant women using IAs. Supported by both previous studies and the current study, measurement of fT4 using an LC-MS/MS reference method is thought to measure fT4 more accurately and could even be recommended for clinical use (22, 39, 40). However, the LC-MS/MS method, preceded by equilibrium dialysis, for measuring fT4 is technically demanding and time- and labor-intensive, making this method for routine clinical laboratories not useful as an alternative measurement method for the measurement of fT4 in samples from pregnant women. Since a proper solution to overcome the aforementioned issues has not been found yet, physicians and laboratory specialists should be aware that matrix effects can cause an fT4 IA bias to avoid drawing the wrong conclusions about thyroid function in pregnant women. Looking for an enduring solution, we encourage manufacturers to improve their fT4 IAs, making the assays more suitable for specific groups with, for example, deviated concentrations of binding globulins. Until then, assay-specific reference intervals for pregnant women, ideally per trimester, are necessary. Reference intervals in pregnant women for different trimesters were established for several immunoassays of Roche, Abbott, Beckman Coulter and Siemens and an ultrafiltration LC-MS/MS method as well (41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51). Studies evaluating reference intervals using the identical IA demonstrated a high consistency with comparable results and a maximal difference of 1–2 pmol/L at the lower or upper reference range (46, 47, 48). However, different fT4 IAs have varying techniques and reagent composition in measuring fT4 concentrations, meaning pregnancy does not influence all different IAs to the same extent as was shown in our study. Therefore, reference intervals for pregnant women need to be established for every IA separately and cannot be used from or recalculated based on other IAs, which has also been highlighted previously by Bliddal et al. (52) and Okosieme et al. (53). Furthermore, patient populations highly differ between countries or regions which could lead to altered reference ranges of thyroid hormone parameters (54). Our study emphasized that every laboratory should determine their own fT4 reference intervals for their pregnant population and IA of use or implement fT4 reference intervals from other laboratories using the same immunoassay and based on a comparable population of pregnant women.
In our study, pregnant women were not selected based on their trimester, so we could not differentiate between inaccuracies in different trimesters during pregnancy. This could be considered a limitation of this study; however, changes in serum matrix of pregnant women are present throughout the whole pregnancy. This study aimed to provide insight into the extent of IA difficulties during pregnancy compared to a candidate conventional reference method, meaning specification per trimester was not essential. Both male and female healthy controls were included in this study. Even though this may seem a limitation, the design of our study did not require perfectly matched groups to demonstrate the accuracy of fT4 IAs in pregnant women. Moreover, time of blood withdrawal and fasting state varied between pregnant women and healthy controls. Although this may be a limitation, literature showed that these circumstances do not influence fT4 concentrations within individuals to a large degree (55). Furthermore, other factors that might influence fT4 concentrations (e.g. smoking status, BMI, ethnicity) were not taken into account in this study (54, 56, 57, 58). Nonetheless, fT4 concentrations in pregnant women were comparable to previous studies, indicating pregnancy was the main component causing altered fT4 concentrations. In this study, we did not include participants with thyroid diseases. In general, literature showed that IAs have more difficulties in measuring fT4 concentrations far above the upper and lower limit of the reference interval (59, 60), meaning fT4 IAs could even be less accurate in pregnant women displaying severe thyroid dysfunction. Therefore, future research should focus on the accuracy of fT4 IAs in this specific group as well. The inclusion of a candidate-reference method and several commonly used IAs are strengths of this study, as the results are highly accurate and can be generalized directly to most clinical laboratories.
In conclusion, our study showed that immunoassays measure falsely high fT4 concentrations in pregnant women compared to healthy controls, leading to an overestimation of thyroid hormone status and providing insight into the extent of immunoassay difficulties during pregnancy by using an fT4 candidate-reference method. Assay-specific fT4 reference intervals for pregnant women should be established to ascertain reliable interpretation, together with an (overall) improvement of the assay by the manufacturers.