madman
Super Moderator
Acquired Hypogonadotropic Hypogonadism Trial in Italy (Lutropin alfa, Human chorionic gonadotropin) | Clincosm
Recruiting. Rec-LH PD and Safety Profile in Hypogonadotropic Hypogonadism Men
STUDY DESCRIPTION
Brief Summary
Objectives: The overall clinical question is whether LH supplementation to men in indication for FSH according to the AIFA note 74, or with HH, will improve spermatogenesis and pregnancy rate (spontaneous or after ART) over FSH alone or FSH+hCG. However, since LH has never been used in men so far, the first, specific object of this study is the assessment of pharmacodynamics and safety profile of LH in HH men. To this end, this study will evaluate the pharmacodynamics and safety profile of recombinant LH (Luveris) and compare the response to Luveris and urinary hCG (Gonasi HP) in HH men. The pharmacodynamics will be assessed primarily for testosterone levels in response to increasing doses of LH and the comparison of the response to a fixed dose of hCG, and later for a more extend steroid profile.
Methods: Multicentre longitudinal, interventional, randomized, open-label, phase II, clinical trial, assessing pharmacodynamics of LH in acquired HH men. The statistical hypothesis is non-inferiority of the highest LH dose employed compared to a fixed hCG dose.
Primary endpoint: serum testosterone levels evaluated by liquid-chromatography, tandem mass spectrometry (LC-MS/MS).
Secondary endpoints: Safety and tolerability as determined by AE reporting, vital signs, and ECG, stereognosis (inhibin B, free testosterone, sex hormone-binding globulin (SHBG), estradiol, whole steroid profile provided by LC-MS/MS), and testicular volume.
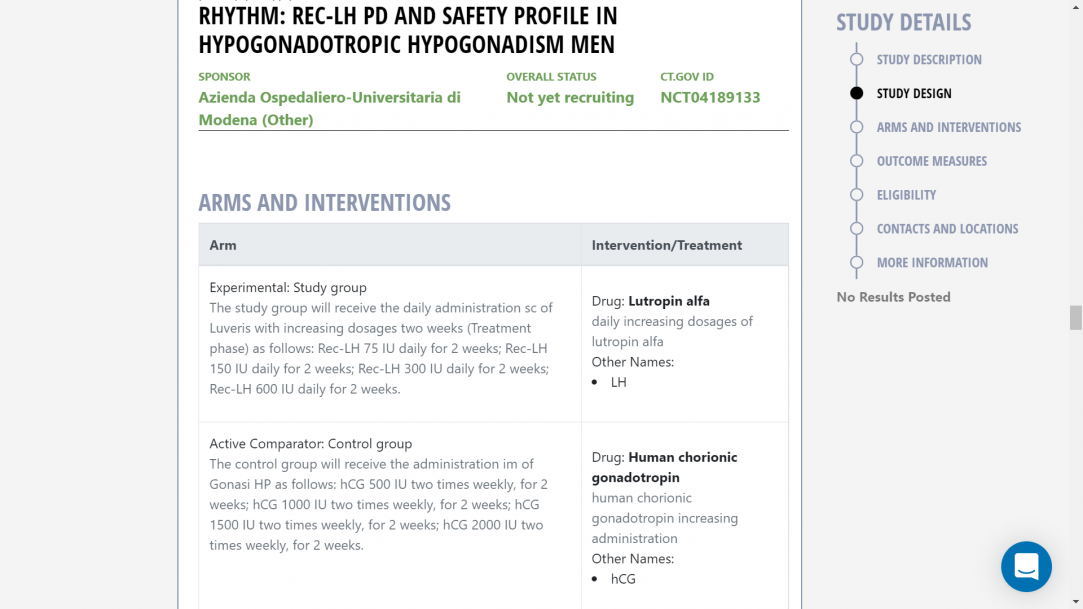
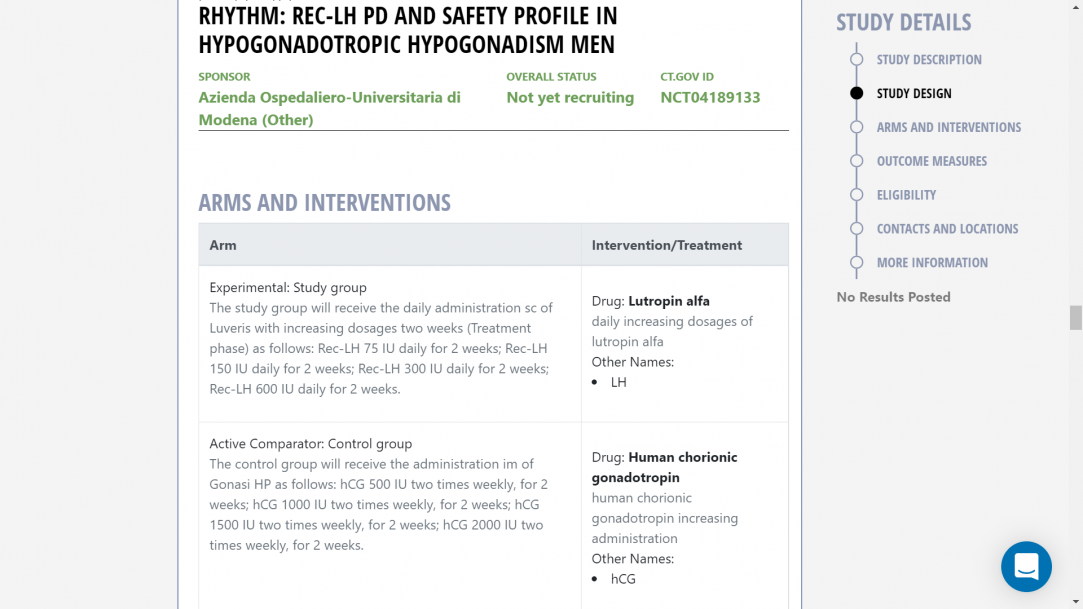
Patients: 32 men with acquired HH, including HH after neurosurgery for tumors or HH due to pituitary adenoma-related mass effect. Patients will be randomized (1:1) according to a permuted-blocks randomization list, to the study group, treated with Luveris (increasing doses at two weekly intervals), or to the control group treated with Gonasi HP (2000 IU twice/week). In the study group, increasing LH dosages will be administered to obtain a testosterone dose-response curve, starting with the minimum expected efficient dose (75 IU/d, sc) for two weeks followed by 150, 225, and 300 IU at the two-weekly intervals, respectively. The control group will be treated by the standard approach, i.e. hCG 2000 IU IM twice-weekly for 8 weeks. Patients will be further followed up for 4 weeks after treatment withdrawal. During the study, the patients will be evaluated two times per week during the treatment phase and every two weeks in the follow-up phase.
AT V1 PATIENTS WILL BE RANDOMIZED INTO TWO DIFFERENT GROUPS:
Study group Control groupThe study group will receive the daily administration sc of Luveris with increasing dosages every two weeks (Treatment phase) as follows:
- Rec-LH 75 IU daily for 2 weeks;
- Rec-LH 150 IU daily for 2 weeks;
- Rec-LH 300 IU daily for 2 weeks;
- Rec-LH 600 IU daily for 2 weeks. The decision to proceed to the next dose level of LH will be made by the Study Team [and the investigator] based on safety, tolerability, and preliminary data obtained in at least 5 participants at the prior dose level. If moderate or severe AE is consistently observed across participants in a cohort or if unacceptable pharmacological effects, reasonably attributable to LH in the opinion of the investigator are observed in more than 15% of the participants in a cohort, then dose escalation will be temporarily halted and no further participants will be dosed until a full safety review of the study has taken place. Relevant reporting and discussion with the Medical Monitor, PI, and the IRB/IEC will take place before the resumption of dosing.