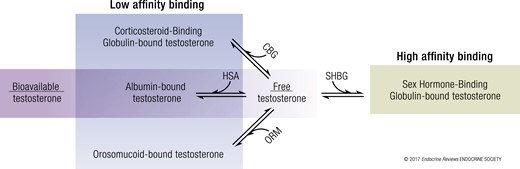
An SHBG sponge analogy is compatible with your other points. I've used a similar reservoir analogy. Think of testosterone as like water being dripped onto the sponge. The important feature is that the sponge saturates, at which point it doesn't interfere with the "flow" of free testosterone—free testosterone being driven exclusively by endogenous production or exogenous dosing. If you double the size of the sponge then the flow is temporarily impaired until the sponge is again saturated. Then free testosterone flows the same as before. A larger sponge means more total testosterone is held, but at steady state this is independent of the flow of free testosterone.The idea of SHBG as a sponge that sucks up large amounts of testosterone and leaves your free T low doesn't seem to be how it really works. The way I understand it, high SHBG makes your meager production (revealed by the free T) look like it's normal or even high when it isn't. The SHBG is basically extending the half-life of the testosterone you've produced and the relationship seems to me alot like ferritin and iron.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Calculate Free Testosterone with TruT by FPT
- Thread starter madman
- Start date
FunkOdyssey
Seeker of Wisdom
An SHBG sponge analogy is compatible with your other points. I've used a similar reservoir analogy. Think of testosterone as like water being dripped onto the sponge. The important feature is that the sponge saturates, at which point it doesn't interfere with the "flow" of free testosterone—free testosterone being driven exclusively by endogenous production or exogenous dosing. If you double the size of the sponge then the flow is temporarily impaired until the sponge is again saturated. Then free testosterone flows the same as before. A larger sponge means more total testosterone is held, but at steady state this is independent of the flow of free testosterone.
This is gold. There is so much misunderstanding around SHBG, it cries out for good analogies. Reddit is full of guys that think they just need to knock down their SHBG somehow to double their free T and don't realize all they're likely to accomplish is a reduction of their total T.
T
tareload
Guest
Fun experiment to try with oxandrolone that confirms above with some liberties taken.This is gold. There is so much misunderstanding around SHBG, it cries out for good analogies. Reddit is full of guys that think they just need to knock down their SHBG somehow to double their free T and don't realize all they're likely to accomplish is a reduction of their total T.

Who's currently on T propionate?
The support is appreciated. Nonetheless, the goal here is to improve our knowledge. That can only occur when new ideas face close scrutiny and survive informed criticism. @DS3 was on point to bring up the subject of direct metabolism of bound testosterone. But his other comments are mainly vague...
T
tareload
Guest

Realistic TRT Recomp Progress
Thanks for sharing but this last entry doesn’t compute for me. This is Labcorp LC/MS for TT and fT with ED or Ultrafiltration? Given your TT and SHBG your FT should be around 50 ng/dL (about 2% fT/TT as your previous results) using Vermeulen method which tracks accurate free T reasonably well...
 forums.t-nation.com
forums.t-nation.com

Public Service Announcement: "Piss Poor" Direct RIA fT measurement (HELP is on the way)
Hey Folks, thought I would update the graph for free T reference ranges based on LC-MS/MS+ED and compare with Labcorp's direct RIA fT reference range. What the hell do you do with with your direct fT measurement if you want to compare with equibrium dialysis reference ranges? Take the Labcorp...
Thanks again @Cataceous for this meaningful sponge analogy. I agree with @Funkodyssey this helps a lot to have a better picture of the whole mechanism.
According to the literature I found, the half-life of SHBG-trapped T is longer, but not terribly much, which means that the sponge is actually also a sink (or a sponge in which T disappears)
half-life of free T: 18 minutes
half-life of SHBG-bound T : 32 minutes

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
what is your opinion?
According to the literature I found, the half-life of SHBG-trapped T is longer, but not terribly much, which means that the sponge is actually also a sink (or a sponge in which T disappears)
half-life of free T: 18 minutes
half-life of SHBG-bound T : 32 minutes

Kinetics of removal of intravenous testosterone pulses in normal men - PubMed
A frequent-sampling strategy comprising an experimental hormone clamp, estimation of hormone concentrations as bound and free moieties, mimicry of physiological pulses, and deconvolution analysis may have utility in estimating the in vivo kinetics of other hormones, substrates, and metabolites.
what is your opinion?
madman
Super Moderator
METHODS AND SYSTEMS FOR THE DIAGNOSIS AND TREATMENT OF SEX HORMONE DISORDERS
 patents.justia.com
patents.justia.com
 patents.justia.com
patents.justia.com

 www.excelmale.com
www.excelmale.com
Ravi Jasuja Inventions, Patents and Patent Applications - Justia Patents Search
USPTO patent applications submitted by and patents granted to Ravi Jasuja
 patents.justia.com
patents.justia.com
US Patent Application for METHODS AND SYSTEMS FOR THE DIAGNOSIS AND TREATMENT OF SEX HORMONE DISORDERS Patent Application (Application #20220206018 issued June 30, 2022) - Justia Patents Search
The technology described herein is directed to the diagnosis and treatment of sex hormone disorders and/or deficiencies, such as estrogen and/or testosterone disorders and/or deficiencies.
 patents.justia.com
patents.justia.com
TruT Free Testosterone Calculator by FPT
Free testosterone calculator by FPT. Improved accuracy of free T calculations.
tru-t.org

Tru T calculator - what is the range?
Labcorp 'normal' range for Free T is 6.8 - 21.5. Obviously, the Tru T calculator produces much different results. For example, on a recent test, my Labcorp Free T was 27.7. The Tru T measure yielded 46. This begs the question, what is the ideal range using the Tru T method. I saw in some...
madman
Super Moderator

What's Coming? Accurate measurement of total and free testosterone levels for the diagnosis of androgen disorders
Accurate measurement of total and free testosterone levels for the diagnosis of androgen disorders (7/2022) Ezgi Caliskan Guzelce, MD, Clinical/ Research Fellow, Francesca Galbiati, MD, Fellow, Anna L. Goldman, MD, Assistant Professor of Medicine, Arijeet K. Gattu, MD, Fellow, Shehzad Basaria...
thanks @madman for all this insightful material.
I wanted to put the eugonadic limit as defined by Vermeulen (total T lower than 11 nmol/L or free T lower than 0.225 nmol/L) with it's formula and show on the same graph the Tru-T median ± 1SD.
we see that Vermeulen is much more sensitive than Tru-T.. are we overestimating the effect of SHBG with Vermeulen and similar formulas?
Last edited:
Hi FunkOdyssey,This is gold. There is so much misunderstanding around SHBG, it cries out for good analogies. Reddit is full of guys that think they just need to knock down their SHBG somehow to double their free T and don't realize all they're likely to accomplish is a reduction of their total T.
I fully agree with you, there is a lot of misunderstanding around SHBG. The sponge analogy is a very good one and shows SHBG from a totally different angle.
Nevertheless, I still think that this sponge consumes a small part of the T (small since the half-life of SHBG-bound-T is longer than free-T). in my mind, SHBG still destroys a part of T, but at small scale.
T
tareload
Guest
Thanks. This will make for some fun discussions!!!!!
madman
Super Moderator
Phase IIB: Development of TruT Algorithm for Commercialization in Androgen Disorders (2022)
 reporter.nih.gov
reporter.nih.gov

TruTTM (v2.0) algorithm
ABSTRACT
Background: Measurement of free testosterone (T) concentrations is indicated in the diagnosis of androgen disorders, including hypogonadism in men; hirsutism, polycystic ovary syndrome (PCOS), and androgenic alopecia in women; pubertal disorders in boys and management of gender-affirming hormone therapies for transgender and gender diverse (TGD) persons. This Phase IIB proposal aims to continue the development of the TruTTM algorithm by validating it in common conditions characterized by altered estradiol (E2), T, and SHBG concentrations and incorporating the interaction of E2 with T for wider commercial adoption in women in whom E2 levels vary greatly across the menstrual cycle and in TGD population.
Approach: This application follows the FDA’s published “Guidance for Industry: Bioanalytical Method Validation”.
The essential parameters to determine the acceptability of a bioanalytical method include its technical performance (accuracy, precision, sensitivity, selectivity, stability, and matrix effects).
Reference ranges should be determined in appropriate human samples.
The analytical method should be validated for the intended use (e.g., determination in conditions of intended use, such as persons with altered E2 and T levels, women with PCOS, TGD persons, etc.).
In studies through the Phase II, we demonstrated that the method has superior performance characteristics and extended the validation of TruTTM algorithm in conditions characterized by altered SHBG concentrations.
*In the proposed Phase IIB studies, we will generate the v2.0 of TruTTM algorithm by incorporating the dynamics of the E2 induced perturbation in free T levels, validate it in men, women, and TGD populations (Aim 1) and deploy HIPAA-compliant, secure integration of the algorithm into electronic medical records (EMR) workflow
*(Aim 2). Future Directions and Commercialization potential: The phase IIB program will enable the pilot commercial deployment of a HIPAA-compliant (FDA registered) platform for commercializing the TruTTM (v2.0) algorithm embedded into electronic medical record (EMR) for wider clinical adoption.
These studies will improve clinical care and advance our fundamental understanding of dynamic regulation of T bioavailability in diverse populations including unrepresented sexual and gender minorities.
Phase IIB: Development of TruT Algorithm for Commercialization in Androgen Disorders (2022)
Project Start Date: 15 September 2014
Project End Date: 31-August-2024
Phase II: Research and Commercialization of TruT Algorithm for Free Testosterone (2018)
 reporter.nih.gov
reporter.nih.gov
Phase II: Research and Commercialization of TruT Algorithm for Free Testosterone (2017)
 reporter.nih.gov
reporter.nih.gov
Novel Algorithm for Free Testosterone Determination (2014)
 reporter.nih.gov
reporter.nih.gov

 www.sciencedirect.com
www.sciencedirect.com
 academic.oup.com
academic.oup.com

 www.excelmale.com
www.excelmale.com

 www.excelmale.com
www.excelmale.com
RePORT 〉 RePORTER
TruTTM (v2.0) algorithm
ABSTRACT
Background: Measurement of free testosterone (T) concentrations is indicated in the diagnosis of androgen disorders, including hypogonadism in men; hirsutism, polycystic ovary syndrome (PCOS), and androgenic alopecia in women; pubertal disorders in boys and management of gender-affirming hormone therapies for transgender and gender diverse (TGD) persons. This Phase IIB proposal aims to continue the development of the TruTTM algorithm by validating it in common conditions characterized by altered estradiol (E2), T, and SHBG concentrations and incorporating the interaction of E2 with T for wider commercial adoption in women in whom E2 levels vary greatly across the menstrual cycle and in TGD population.
Approach: This application follows the FDA’s published “Guidance for Industry: Bioanalytical Method Validation”.
The essential parameters to determine the acceptability of a bioanalytical method include its technical performance (accuracy, precision, sensitivity, selectivity, stability, and matrix effects).
Reference ranges should be determined in appropriate human samples.
The analytical method should be validated for the intended use (e.g., determination in conditions of intended use, such as persons with altered E2 and T levels, women with PCOS, TGD persons, etc.).
In studies through the Phase II, we demonstrated that the method has superior performance characteristics and extended the validation of TruTTM algorithm in conditions characterized by altered SHBG concentrations.
*In the proposed Phase IIB studies, we will generate the v2.0 of TruTTM algorithm by incorporating the dynamics of the E2 induced perturbation in free T levels, validate it in men, women, and TGD populations (Aim 1) and deploy HIPAA-compliant, secure integration of the algorithm into electronic medical records (EMR) workflow
*(Aim 2). Future Directions and Commercialization potential: The phase IIB program will enable the pilot commercial deployment of a HIPAA-compliant (FDA registered) platform for commercializing the TruTTM (v2.0) algorithm embedded into electronic medical record (EMR) for wider clinical adoption.
These studies will improve clinical care and advance our fundamental understanding of dynamic regulation of T bioavailability in diverse populations including unrepresented sexual and gender minorities.
Phase IIB: Development of TruT Algorithm for Commercialization in Androgen Disorders (2022)
Project Start Date: 15 September 2014
Project End Date: 31-August-2024
Phase II: Research and Commercialization of TruT Algorithm for Free Testosterone (2018)
RePORT 〉 RePORTER
Phase II: Research and Commercialization of TruT Algorithm for Free Testosterone (2017)
RePORT 〉 RePORTER
Novel Algorithm for Free Testosterone Determination (2014)
RePORT 〉 RePORTER

A multi-step, dynamic allosteric model of testosterone's binding to sex hormone binding globulin
Circulating free testosterone (FT) levels have been used widely in the diagnosis and treatment of hypogonadism in men. Due to experimental complexitie…
 www.sciencedirect.com
www.sciencedirect.com

A Reappraisal of Testosterone’s Binding in Circulation: Physiological and Clinical Implications
This appraisal of the dynamics of testosterone binding to proteins and of the free hormone hypothesis offers guidance for the application of free testosterone i

Allosterically coupled multi-site binding of T to human serum albumin
Allosterically coupled multi-site binding of testosterone to human serum albumin Abhilash Jayaraj, Heidi A. Schwanz, Daniel J. Spencer, Shalender Bhasin, James A. Hamilton, B. Jayaram, Anna L. Goldman, Meenakshi Krishna, Maya Krishnan, Aashay Shah, Zhendong Jin, Eileen Krenzel, Sashi N. Nair...

The Dynamics of Allosteric Binding of Estradiol to SHBG
Estradiol induces Allosteric Coupling and Partitioning of Sex Hormone Binding Globulin Monomers Among Conformational States Ravi Jasuja, Daniel Spencer, Abhilash Jayaraj, Liming Peng, Meenakshi Krishna, Brian Lawney, Priyank Patel, B. Jayaram, Kelly M. Thayer, David L. Beveridge, Shalender...
madman
Super Moderator
XYone Therapeutics – XYone Therapeutics
 xyonetx.com
xyonetx.com
Endocrine Science
XYone is solving the problems present in the clinical treatment of hormonal disorders such as hypogonadism (Low testosterone condition), Hypothyroidism (Low Thyroxine condition) & other hormonal deficiencies. These typically. have therapeutic solutions that have poor delivery (pharmacokinetics) and almost no personalized or rational dosing. Through our research, we are creating the first-in-class, programmable release, aqueous, nano/microparticle formulation for testosterone and other hormones where we can program the delivery to create solutions which go from the current one size fit all, empirical treatments to personalized, rational dosing regimens.
Our first focus is Testosterone & we are proud to announce that our work is being supported by NIH/SBIR grants and we are partnering with the leading institutions in the world to bring our research to the clinic including Brigham & Women’s Hospital, Mayo Clinic, Center for Disease Control, and Karolinka Institute and others.
Accurate measurement of Androgens
Definitive diagnosis and rational management of androgen disorders currently face a number of challenges.
*Our patent-protected, novel TruT™ companion diagnostic framework provides accurate determination of free testosterone concentrations. This algorithm is based on experimental data demonstrating that testosterone’s binding to SHBG is a multi-step process involving an allosteric interaction between the two binding sites on the SHBG dimer. Estimates of free testosterone derived incorporating the allosteric coupling of SHBG monomers within the dimer provide accurate determination of free testosterone without systematic deviation from values obtained using equilibrium dialysis.
*Ongoing development is focused around continued study and validation in common conditions characterized by altered binding protein concentrations. Further, the incorporation of estradiol interactions will allow for wider adoption in women where estradiol levels vary greatly across the menstrual cycle. Because hyperandrogenism in women is the second most frequent indication for free testosterone determination, understanding the competitive binding and displacement dynamics is important for proper diagnosis in both healthy menstruating women and women with hyperandrogenic disorders, such as PCOS.
Through collaborations and partnerships, the TruT™ platform presents a unique opportunity to aggregate large volumes of data and metadata across diverse populations, ultimately enabling deeper understanding of the basis of androgen disorders and other conditions.
Formulation Development
Problems with current androgen/hormonal therapies
Hormonal deficiency supplementation therapies are characterized by absence of improved formulations with optimal PK profiles and patient-friendly dosing. Our first candidate is Testosterone replacement therapy (TRT).
Testosterone replacement therapy (TRT) is indicated for the treatment of androgen deficiency in hypogonadal men. The US Food and Drug Administration (FDA) has approved several testosterone formulations for the treatment of hypogonadism in men, including injectable testosterone esters, transdermal testosterone patches, transdermal testosterone gels, and solution, buccal adhesive testosterone tablets, intranasal testosterone, and testosterone pellets & recently oral pills. However, each of these modes of testosterone delivery has significant drawbacks.
A feature common to the currently available delivery methods is the high variability in bioavailable testosterone as patients vary between super- and sub-therapeutic levels. This results in undesirable side effects and high rates of treatment discontinuation. These side effects include large fluctuations in mood and sexual function. In addition, treatment-specific side effects are noted, such as inadvertent contact transfer associated with testosterone gel formulations; women and children are particularly susceptible to the significant side effects of cross-contamination.
Thus, there is an unmet clinical need for improved formulations for testosterone replacement therapy (TRT). The limitations of the currently available testosterone formulations have stimulated tremendous interest in developing long-acting delivery systems that can provide uniform circulating levels of testosterone in the target therapeutic range.
nTc Nano/microparticle Formulation
To address the shortcomings of currently available testosterone formulations, the FPT team is in development of an (nTc) nano/microparticle formulation for sustained, consistent release. This novel next-generation formulation offers the promise of superior pharmacokinetics, uniform delivery of testosterone sustained for four weeks or greater, improved adherence, and treatment outcomes for patients seeking testosterone replacement therapy.
The nTc is a novel proprietary nanoparticle formulation that utilizes FDA-approved biocompatible polymeric materials such as poly (lactic-co-glycolic acid), poly (lactic acid), poly (glycolic acid), poly-l- (glutamic acid), and amphiphilic components. This aqueous formulation eliminates the oil depot-related adverse effects, and as shown in preliminary results, delivers testosterone uniformly without the burst release.
Personalization of Hormonal Treatments
We will be able to combine our research in both diagnostics as well as formulations to personalize the treatment of patients uniquely and precisely. Current clinical practice needs multiple office visits as well as lab visits to empirically titrate the correct dosage for a patient. This process needs to be repeated regularly as patient condition changes over time and is a major contributor towards poor adherence and compliance by patients and resulting in suboptimal clinical outcomes. We plan to develop personalized, finely calibrated dosimeters which will enable physicians to accurately determine the dosage for each individual.
T
tareload
Guest
Doc, where'd my GAINZ go???View attachment 27693XYone Therapeutics – XYone Therapeutics
xyonetx.com
View attachment 27694
Endocrine Science
XYone is solving the problems present in the clinical treatment of hormonal disorders such as hypogonadism (Low testosterone condition), Hypothyroidism (Low Thyroxine condition) & other hormonal deficiencies. These typically. have therapeutic solutions that have poor delivery (pharmacokinetics) and almost no personalized or rational dosing. Through our research, we are creating the first-in-class, programmable release, aqueous, nano/microparticle formulation for testosterone and other hormones where we can program the delivery to create solutions which go from the current one size fit all, empirical treatments to personalized, rational dosing regimens.
Our first focus is Testosterone & we are proud to announce that our work is being supported by NIH/SBIR grants and we are partnering with the leading institutions in the world to bring our research to the clinic including Brigham & Women’s Hospital, Mayo Clinic, Center for Disease Control, and Karolinka Institute and others.
Accurate measurement of Androgens
Definitive diagnosis and rational management of androgen disorders currently face a number of challenges.
*Our patent-protected, novel TruT™ companion diagnostic framework provides accurate determination of free testosterone concentrations. This algorithm is based on experimental data demonstrating that testosterone’s binding to SHBG is a multi-step process involving an allosteric interaction between the two binding sites on the SHBG dimer. Estimates of free testosterone derived incorporating the allosteric coupling of SHBG monomers within the dimer provide accurate determination of free testosterone without systematic deviation from values obtained using equilibrium dialysis.
*Ongoing development is focused around continued study and validation in common conditions characterized by altered binding protein concentrations. Further, the incorporation of estradiol interactions will allow for wider adoption in women where estradiol levels vary greatly across the menstrual cycle. Because hyperandrogenism in women is the second most frequent indication for free testosterone determination, understanding the competitive binding and displacement dynamics is important for proper diagnosis in both healthy menstruating women and women with hyperandrogenic disorders, such as PCOS.
Through collaborations and partnerships, the TruT™ platform presents a unique opportunity to aggregate large volumes of data and metadata across diverse populations, ultimately enabling deeper understanding of the basis of androgen disorders and other conditions.
View attachment 27695
Formulation Development
Problems with current androgen/hormonal therapies
Hormonal deficiency supplementation therapies are characterized by absence of improved formulations with optimal PK profiles and patient-friendly dosing. Our first candidate is Testosterone replacement therapy (TRT).
Testosterone replacement therapy (TRT) is indicated for the treatment of androgen deficiency in hypogonadal men. The US Food and Drug Administration (FDA) has approved several testosterone formulations for the treatment of hypogonadism in men, including injectable testosterone esters, transdermal testosterone patches, transdermal testosterone gels and solution, buccal adhesive testosterone tablets, intranasal testosterone, and testosterone pellets & recently oral pills. However, each of these modes of testosterone delivery has significant drawbacks.
A feature common to the currently available delivery methods is the high variability in bioavailable testosterone as patients vary between super- and sub-therapeutic levels. This results in undesirable side effects and high rates of treatment discontinuation. These side effects include large fluctuations in mood and sexual function. In addition, treatment-specific side effects are noted, such as inadvertent contact transfer associated with testosterone gel formulations; women and children are particularly susceptible to the significant side effects of cross-contamination.
Thus, there is an unmet clinical need for improved formulations for testosterone replacement therapy (TRT). The limitations of the currently available testosterone formulations have stimulated tremendous interest in developing long-acting delivery systems that can provide uniform circulating levels of testosterone in the target therapeutic range.
nTc Nano/microparticle Formulation
To address the shortcomings of currently available testosterone formulations, the FPT team is in development of an (nTc) nano/microparticle formulation for sustained, consistent release. This novel next-generation formulation offers the promise of superior pharmacokinetics, uniform delivery of testosterone sustained for four weeks or greater, improved adherence, and treatment outcomes for patients seeking testosterone replacement therapy.
The nTc is a novel proprietary nanoparticle formulation that utilizes FDA-approved biocompatible polymeric materials such as poly (lactic-co-glycolic acid), poly (lactic acid), poly (glycolic acid), poly-l- (glutamic acid), and amphiphilic components. This aqueous formulation eliminates the oil depot-related adverse effects, and as shown in preliminary results, delivers testosterone uniformly without the burst release.
Personalization of Hormonal Treatments
We will be able to combine our research in both diagnostics as well as formulations to personalize the treatment of patients uniquely and precisely. Current clinical practice needs multiple office visits as well as lab visits to empirically titrate the correct dosage for a patient. This process needs to be repeated regularly as patient condition changes over time and is a major contributor towards poor adherence and compliance by patients and resulting in suboptimal clinical outcomes. We plan to develop personalized, finely calibrated dosimeters which will enable physicians to accurately determine the dosage for each individual.
Doc will have those instantaneous TT/fT levels popping up on her smart phone.
T
tareload
Guest
Head to head Quest and Labcorp LCMS+ED results against Vermeulen and TruT calculators coming soon. Guess who will dominate?
T
tareload
Guest
FUN WITH TT/FT Blood Work Part I (dedicated to @madman for all his efforts posting on this site and to get my steak dinner LOL):
Ok, I know you all have been sitting on the edge of your seats. Head to Head Quest vs Labcorp.
24 hr post injection on TC:TP (4:1) at 60 mg twice weekly (120 mg/week total ester). 6 weeks with this protocol before blood work up from 100 mg/week
Quest (thanks to @Nelson Vergel 's Discounted Labs service):
 testdirectory.questdiagnostics.com
testdirectory.questdiagnostics.com


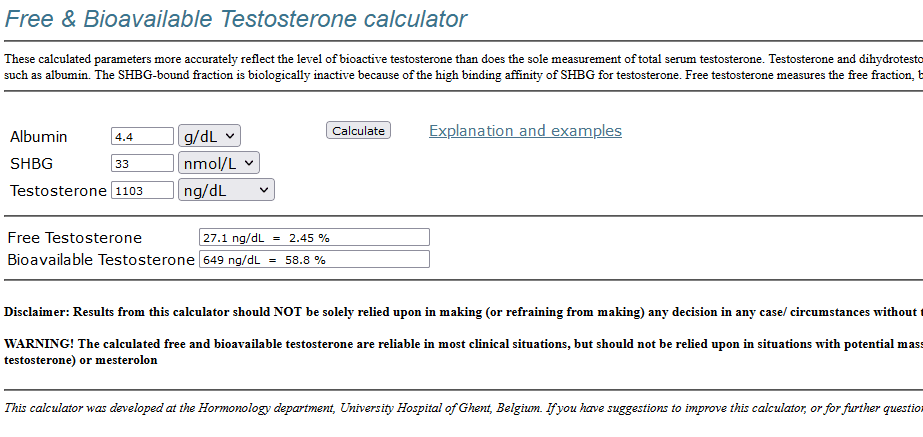
Quest TT: 1100 ng/dl (MS)
Quest FT: 24.5 ng/dl (equilibrium dialysis)
calculated FT (Vermeulen):
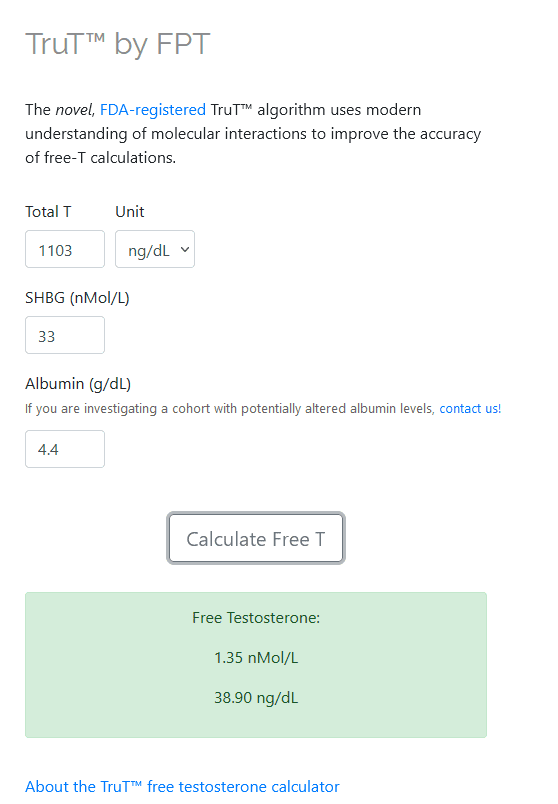
calculated FT (TruT):
Using my simple heuristic of taking 10% off the Vermeulen calc FT number:
adj CFTV = 0.9 * 27.1 ng/dl = 24.4 ng/dl
Quest FT = 24.5 ng/dl
Coming up...detailed comparison using Labcorp with an extra twist.
Ok, I know you all have been sitting on the edge of your seats. Head to Head Quest vs Labcorp.
24 hr post injection on TC:TP (4:1) at 60 mg twice weekly (120 mg/week total ester). 6 weeks with this protocol before blood work up from 100 mg/week
Quest (thanks to @Nelson Vergel 's Discounted Labs service):
Quest Diagnostics: Test Directory
Quest TT: 1100 ng/dl (MS)
Quest FT: 24.5 ng/dl (equilibrium dialysis)
calculated FT (Vermeulen):
calculated FT (TruT):
Using my simple heuristic of taking 10% off the Vermeulen calc FT number:
adj CFTV = 0.9 * 27.1 ng/dl = 24.4 ng/dl
Quest FT = 24.5 ng/dl
Coming up...detailed comparison using Labcorp with an extra twist.
Last edited by a moderator:
T
tareload
Guest
FUN WITH TT/FT Blood Work Part II (dedicated to @madman for all his efforts posting on this site and to get my steak dinner LOL):
Labcorp:
I have outdone myself here as I got blood drawn and had the technician order BOTH:
 www.labcorp.com
www.labcorp.com
AND
 www.labcorp.com
www.labcorp.com
This allows me to get two different labs to measure TT via LCMS (with claimed CDC HoST status) plus FT by equilibrium dialysis and the direct analog enzyme immunoassay (aka direct FT test).
BEHOLD:
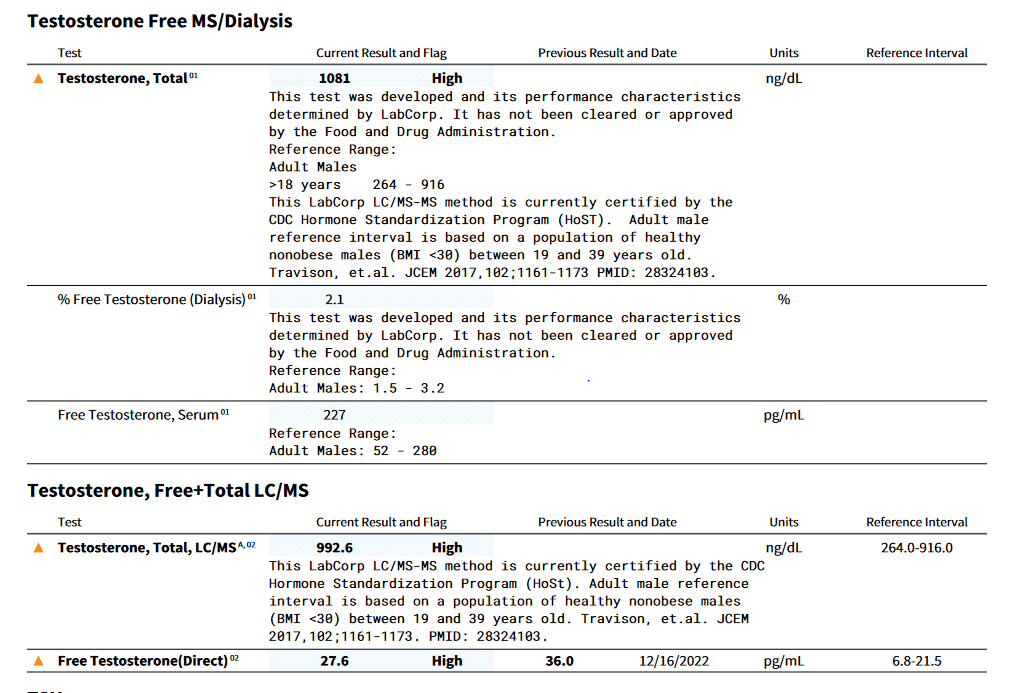

First, let's use the Esoterix lab as the standard (arbitrary) even though both Labcorp's Esoterix Lab and Burlington Lab have HoST certification:
% difference = (1081-992.6)/1081 *100 = 8.2% difference between the two CDC HoST certified labs.
Esoterix sounds more impressive than Burlington so it has that going for it.
Second, Labcorp FT by equilibrium dialysis = 22.7 ng/dl. Remember Quest FT by ED on same sample was 24.5 ng/dl (see above). So regardless of the different reference ranges both assays have very similar FT result. Labcorp's Esoterix TT by MS and Quest TT by MS are also very close (1081 vs 1103 ng/dl).
Third, what does the Labcorp direct FT test tell us. Using the handy transfer function tool I detailed in another thread:
Adjusted FT (direct) = 27.6 pg/ml * 7 / 10 = 19.3 ng/dl
Quite a bit lower than either Labcorp or Quest's FT by ED but in the ball park.
% difference = (24.5-19.3)/24.5 = 21%
So to summarize, on the same blood sample roughly approximating peak blood levels 24 hours after injection for TC (of course off on the TP part):
RIP TruT (CFTZ) v1.0:

 www.excelmale.com
www.excelmale.com
See this thread for more information than you probably want. Now you don't have to post the question about which free T calculator to use until Madman posts up the next Phase 2 validation results for TruT.
Now let's see how many likes these posts get. Come on I need the dopamine hits. Congratulations if you made it all the way through both posts.
Labcorp:
I have outdone myself here as I got blood drawn and had the technician order BOTH:
500726: Testosterone, Free, Mass Spectrometry/Equilibrium Dialysis (Endocrine Sciences) | Labcorp
Labcorp test details for Testosterone, Free, Mass Spectrometry/Equilibrium Dialysis (Endocrine Sciences)
AND
070195: Testosterone, Free, Direct With Total Testosterone, LC/MS-MS | Labcorp
Labcorp test details for Testosterone, Free, Direct With Total Testosterone, LC/MS-MS
This allows me to get two different labs to measure TT via LCMS (with claimed CDC HoST status) plus FT by equilibrium dialysis and the direct analog enzyme immunoassay (aka direct FT test).
BEHOLD:
First, let's use the Esoterix lab as the standard (arbitrary) even though both Labcorp's Esoterix Lab and Burlington Lab have HoST certification:
% difference = (1081-992.6)/1081 *100 = 8.2% difference between the two CDC HoST certified labs.
Esoterix sounds more impressive than Burlington so it has that going for it.
Second, Labcorp FT by equilibrium dialysis = 22.7 ng/dl. Remember Quest FT by ED on same sample was 24.5 ng/dl (see above). So regardless of the different reference ranges both assays have very similar FT result. Labcorp's Esoterix TT by MS and Quest TT by MS are also very close (1081 vs 1103 ng/dl).
Third, what does the Labcorp direct FT test tell us. Using the handy transfer function tool I detailed in another thread:
Adjusted FT (direct) = 27.6 pg/ml * 7 / 10 = 19.3 ng/dl
Quite a bit lower than either Labcorp or Quest's FT by ED but in the ball park.
% difference = (24.5-19.3)/24.5 = 21%
So to summarize, on the same blood sample roughly approximating peak blood levels 24 hours after injection for TC (of course off on the TP part):
- TT (Quest MS) = 1103 ng/dl
- TT (Labcorp Esoterix LCMS) = 1081 ng/dl
- TT (Labcorp Burlington (LCMS) = 993 ng/dl
- FT (Quest ED) = 24.5 ng/dl
- FT (Labcorp Esoterix ED) = 22.7 ng/dl
- FT (adj direct FT EIA) = 19.3 ng/dl
- cFTV (Vermeulen) ~ 27.1 ng/dl
- adjusted cFTV (90% of CFTV) ~ 24.4 ng/dl
- TruT ~ 38.9 ng/dl
- Both Labcorp and Quest agree pretty well on measured FT via ED even through reference ranges are quite different as we discussed before).
- The adjusted direct FT still gets you in the ballpark although the low price of the FT by ED using @Nelson Vergel's Discounted Labs make it stupid easy to get FT by ED with very low cost.
- Interlab variability with the two Labcorp HoST sites was ~8% for my blood sample.
- TruT calculator as it currently exists on the website SUCKS unless Labcorp and Quest are both measuring FT wrong. Neither Quest nor Labcorp line up with the training set TruT claims matches their calculated values. If Labcorp and Quest are both full of sh*t then why are we telling guys to keep ordering these tests LOL?
RIP TruT (CFTZ) v1.0:

Reference Intervals for Free Testosterone in Adult Men Measured Using a Standardized Equilibrium Dialysis Procedure
... I am too impatient today to wait for the next paper so here you go. You got it here first on @Nelson Vergel 's ExcelMale. Given the Tru-T website locks you down after a few tries I didn't fill out the whole table but covered the boundaries: ... Very nice work! Shows why cfTV sometimes...
See this thread for more information than you probably want. Now you don't have to post the question about which free T calculator to use until Madman posts up the next Phase 2 validation results for TruT.
Now let's see how many likes these posts get. Come on I need the dopamine hits. Congratulations if you made it all the way through both posts.
Last edited by a moderator:
T
tareload
Guest
Prettied up summary table:
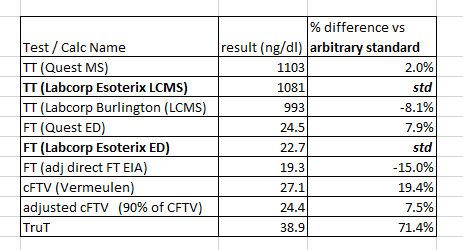
Test / Calc Name | result (ng/dl) | % difference vs arbitrary standard |
TT (Quest MS) | 1103 | 2.0% |
TT (Labcorp Esoterix LCMS) | 1081 | std |
TT (Labcorp Burlington (LCMS) | 993 | -8.1% |
FT (Quest ED) | 24.5 | 7.9% |
FT (Labcorp Esoterix ED) | 22.7 | std |
FT (adj direct FT EIA) | 19.3 | -15.0% |
cFTV (Vermeulen) | 27.1 | 19.4% |
adjusted cFTV (90% of CFTV) | 24.4 | 7.5% |
TruT | 38.9 | 71.4% |
...
...
- TruT ~ 38.9 ng/dl
For grins, take the Tru-T number of 38.9 ng/dL and do a linear mapping from its healthy normal range of 16-31 ng/dL to the cFTV range I use as healthy and normal, 10-20 ng/dL. I get 25.3 ng/dL, which when multiplied by the 0.9 correction factor yields 22.7 ng/dL, coincidentally a direct hit on the Labcorp FT. I think Tru-T is usable if you compare it to its own reference range and SHBG is not very high or low. Tru-T seems to perform worse than cfTV at more extreme SHBG, and of course the absolute numbers are out there.
Basically the LabCorp and Quest FT by dialysis agree, and the cFTV and Labcorp Direct FT also agree but are a bit higher than the dialysis tests and need a depressing factor less than 1.0. All that for someone with a normal SHBG.
Trying to map the values based on "normal ranges" for each test is pontless - they use different samples that are not representative of the healthy population at all. In order to get such samples they have to sample the healthy population randomly and force the selected participants to do a test. Achieving that is not possible so the "representative" samples vary by the lab and so are the "normal ranges".
Trying to map the values based on "normal ranges" for each test is pontless - they use different samples that are not representative of the healthy population at all. In order to get such samples they have to sample the healthy population randomly and force the selected participants to do a test. Achieving that is not possible so the "representative" samples vary by the lab and so are the "normal ranges".
Online statistics
- Members online
- 11
- Guests online
- 6
- Total visitors
- 17
Totals may include hidden visitors.
© Copyright 2020 ExcelMale
















